Auditory analysis of xeroderma pigmentosum 1971-2012: hearing function, sun sensitivity and DNA repair predict neurological degeneration
- PMID: 23365097
- PMCID: PMC3562077
- DOI: 10.1093/brain/aws317
Auditory analysis of xeroderma pigmentosum 1971-2012: hearing function, sun sensitivity and DNA repair predict neurological degeneration
Abstract
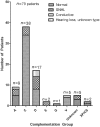
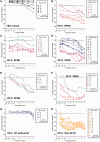
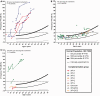
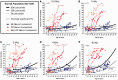
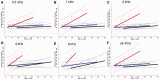
To assess the role of DNA repair in maintenance of hearing function and neurological integrity, we examined hearing status, neurological function, DNA repair complementation group and history of acute burning on minimal sun exposure in all patients with xeroderma pigmentosum, who had at least one complete audiogram, examined at the National Institutes of Health from 1971 to 2012. Seventy-nine patients, aged 1-61 years, were diagnosed with xeroderma pigmentosum (n = 77) or xeroderma pigmentosum/Cockayne syndrome (n = 2). A total of 178 audiograms were included. Clinically significant hearing loss (>20 dB) was present in 23 (29%) of 79 patients. Of the 17 patients with xeroderma pigmentosum-type neurological degeneration, 13 (76%) developed hearing loss, and all 17 were in complementation groups xeroderma pigmentosum type A or type D and reported acute burning on minimal sun exposure. Acute burning on minimal sun exposure without xeroderma pigmentosum-type neurological degeneration was present in 18% of the patients (10/55). Temporal bone histology in a patient with severe xeroderma pigmentosum-type neurological degeneration revealed marked atrophy of the cochlear sensory epithelium and neurons. The 19-year mean age of detection of clinically significant hearing loss in the patients with xeroderma pigmentosum with xeroderma pigmentosum-type neurological degeneration was 54 years younger than that predicted by international norms. The four frequency (0.5/1/2/4 kHz) pure-tone average correlated with degree of neurodegeneration (P < 0.001). In patients with xeroderma pigmentosum, aged 4-30 years, a four-frequency pure-tone average ≥10 dB hearing loss was associated with a 39-fold increased risk (P = 0.002) of having xeroderma pigmentosum-type neurological degeneration. Severity of hearing loss parallels neurological decline in patients with xeroderma pigmentosum-type neurological degeneration. Audiometric findings, complementation group, acute burning on minimal sun exposure and age were important predictors of xeroderma pigmentosum-type neurological degeneration. These results provide evidence that DNA repair is critical in maintaining neurological integrity of the auditory system.
Figures


References
-
- American National Standard Institute. New York: American National Standards Institute; 2003. Maximum permissible ambient noise levels for audiometric test rooms (Standard S3.1) (ANSI. S3.1-1999)
-
- American National Standards Institute. New York: American National Standards Institute; 2004. American national standard specification for audiometers (Standard S3.6) (ANSI. S3.6-1996)
-
- Anttinen A, Koulu L, Nikoskelainen E, Portin R, Kurki T, Erkinjuntti M, et al. Neurological symptoms and natural course of xeroderma pigmentosum. Brain. 2008;131:1979–89. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

