New insights into genotype-phenotype correlations for the doublecortin-related lissencephaly spectrum
- PMID: 23365099
- PMCID: PMC3562079
- DOI: 10.1093/brain/aws323
New insights into genotype-phenotype correlations for the doublecortin-related lissencephaly spectrum
Abstract
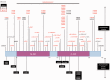
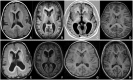
X-linked isolated lissencephaly sequence and subcortical band heterotopia are allelic human disorders associated with mutations of doublecortin (DCX), giving both familial and sporadic forms. DCX encodes a microtubule-associated protein involved in neuronal migration during brain development. Structural data show that mutations can fall either in surface residues, likely to impair partner interactions, or in buried residues, likely to impair protein stability. Despite the progress in understanding the molecular basis of these disorders, the prognosis value of the location and impact of individual DCX mutations has largely remained unclear. To clarify this point, we investigated a cohort of 180 patients who were referred with the agyria-pachygyria subcortical band heterotopia spectrum. DCX mutations were identified in 136 individuals. Analysis of the parents' DNA revealed the de novo occurrence of DCX mutations in 76 cases [62 of 70 females screened (88.5%) and 14 of 60 males screened (23%)], whereas in the remaining cases, mutations were inherited from asymptomatic (n = 14) or symptomatic mothers (n = 11). This represents 100% of families screened. Female patients with DCX mutation demonstrated three degrees of clinical-radiological severity: a severe form with a thick band (n = 54), a milder form (n = 24) with either an anterior thin or an intermediate thickness band and asymptomatic carrier females (n = 14) with normal magnetic resonance imaging results. A higher proportion of nonsense and frameshift mutations were identified in patients with de novo mutations. An analysis of predicted effects of missense mutations showed that those destabilizing the structure of the protein were often associated with more severe phenotypes. We identified several severe- and mild-effect mutations affecting surface residues and observed that the substituted amino acid is also critical in determining severity. Recurrent mutations representing 34.5% of all DCX mutations often lead to similar phenotypes, for example, either severe in sporadic subcortical band heterotopia owing to Arg186 mutations or milder in familial cases owing to Arg196 mutations. Taken as a whole, these observations demonstrate that DCX-related disorders are clinically heterogeneous, with severe sporadic and milder familial subcortical band heterotopia, each associated with specific DCX mutations. There is a clear influence of the individual mutated residue and the substituted amino acid in determining phenotype severity.
Figures




References
-
- Aigner L, Fluegel D, Dietrich J, Ploetz S, Winkler J. Isolated lissencephaly sequence and double-cortex syndrome in a German family with a novel doublecortin mutation. Neuropediatrics. 2000;31:195–8. - PubMed
-
- Aigner L, Uyanik G, Couillard-Despres S, Ploetz S, Wolff G, Morris-Rosendahl D, et al. Somatic mosaicism and variable penetrance in doublecortin-associated migration disorders. Neurology. 2003;60:329–32. - PubMed
-
- Barkovich AJ, Guerrini R, Battaglia G, Kalifa G, N'Guyen T, Parmeggiani A, et al. Band heterotopia: correlation of outcome with magnetic resonance imaging parameters. Ann Neurol. 1994;36:609–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

