Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination
- PMID: 23365216
- PMCID: PMC6619133
- DOI: 10.1523/JNEUROSCI.3334-12.2013
Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination
Abstract
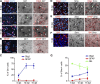
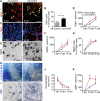
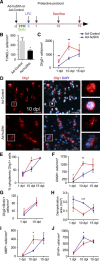
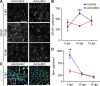
The morphogen Sonic Hedgehog (Shh) controls the generation of oligodendrocyte (OLs) during embryonic development and regulates OL production in adulthood in the cortex and corpus callosum. The roles of Shh in CNS repair following lesions associated with demyelinating diseases are still unresolved. Here, we address this issue by using a model of focal demyelination induced by lysolecithin in the corpus callosum of adult mice. Shh transcripts and protein were not detected in control animals but were upregulated in a time-dependent manner in the oligodendroglial lineage within the lesion. We report an increased transcription of Shh target genes suggesting a broad reactivation of the Shh pathway. We show that the adenovirus-mediated transfer of Shh into the lesioned brain results in the attenuation of the lesion extent with the increase of OL progenitor cells (OPCs) and mature myelinating OL numbers due to survival, proliferation, and differentiation activities as well as the decrease of astrogliosis and macrophage infiltration. Furthermore, the blocking of Shh signaling during the lesion, using its physiological antagonist, Hedgehog interacting protein, results in a decrease of OPC proliferation and differentiation, preventing repair. Together, our findings identify Shh as a necessary factor playing a positive role during demyelination and indicate that its signaling activation stands as a potential therapeutic approach for myelin diseases.
Figures




References
-
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. - PubMed
-
- Angot E, Loulier K, Nguyen-Ba-Charvet KT, Gadeau AP, Ruat M, Traiffort E. Chemoattractive activity of sonic hedgehog in the adult subventricular zone modulates the number of neural precursors reaching the olfactory bulb. Stem Cells. 2008;26:2311–2320. - PubMed
-
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
