Interaction between orbital prefrontal and rhinal cortex is required for normal estimates of expected value
- PMID: 23365223
- PMCID: PMC3725547
- DOI: 10.1523/JNEUROSCI.3605-12.2013
Interaction between orbital prefrontal and rhinal cortex is required for normal estimates of expected value
Abstract
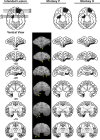
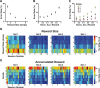
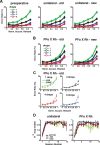
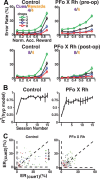
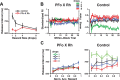
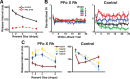
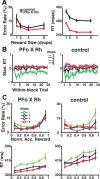
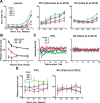
Predicting and valuing potential rewards requires integrating sensory, associative, and contextual information with subjective reward preferences. Previous work has identified regions in the prefrontal cortex and medial temporal lobe believed to be important for each of these functions. For example, activity in the orbital prefrontal cortex (PFo) encodes the specific sensory properties of and preferences for rewards, while activity in the rhinal cortex (Rh) encodes stimulus-stimulus and stimulus-reward associations. Lesions of either structure impair the ability to use visual cues or the history of previous reinforcement to value expected rewards. These areas are linked via reciprocal connections, suggesting it might be their interaction that is critical for estimating expected value. To test this hypothesis, we interrupted direct, intra-hemispheric PFo-Rh interaction in monkeys by performing crossed unilateral ablations of these regions (functional disconnection). We asked whether this circuit is crucial primarily for cue-reward association or for estimating expected value per se, by testing these monkeys, as well as intact controls, on tasks in which expected value was either visually cued or had to be inferred from block-wise changes in reward size in uncued trials. Functional disconnection significantly affected performance in both tasks. Specifically, monkeys with functional disconnection showed less of a difference in error rates and reaction times across reward sizes, in some cases behaving as if they expected rewards to be of equal magnitude. These results support a model whereby information about rewards signaled in PFo is combined with associative and contextual information signaled within Rh to estimate expected value.
Figures


References
-
- Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysiol. 1996;75:1061–1073. - PubMed
-
- Brown MW, Wilson FA, Riches IP. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Res. 1987;409:158–162. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
