Chronic inactivation of a neural circuit enhances LTP by inducing silent synapse formation
- PMID: 23365245
- PMCID: PMC3804283
- DOI: 10.1523/JNEUROSCI.3880-12.2013
Chronic inactivation of a neural circuit enhances LTP by inducing silent synapse formation
Abstract
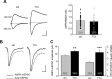
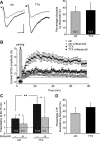
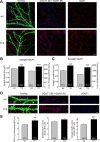
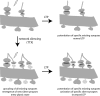
Chronic inactivation of a neural network is known to induce homeostatic upregulation of synaptic strength, a form of synaptic plasticity that differs from Hebbian-type synaptic plasticity in that it is not input-specific, but involves all synapses of an individual neuron. However, it is unclear how homeostatic and Hebbian synaptic plasticity interact in the same neuron. Here we show that long-term potentiation (LTP) at Schaffer collateral-CA1 synapses is greatly enhanced in cultured mouse hippocampal slices after chronic (60 h) network-activity blockade with tetrodotoxin (TTX). This increase in LTP is not due to an altered synaptic NMDA receptor composition or presynaptic function. Instead, we found that silencing neural network activity not only increases the abundance of both AMPA and NMDA receptors at existing synapses as previously described, but also promotes the presence of new glutamatergic synapses that contain only NMDA receptors-a class of synapses that are functionally silent due to the absence of AMPA receptors. Induction of LTP in TTX-treated neurons leads to insertion of AMPA receptors into the silent synapses, thereby "switching on" these silent synapses, which produces the observed enhancement of LTP magnitude. Our findings suggest that homeostatic synaptic plasticity manifests not only in the adjustment of the strength of existing synapses, but also in the modulation of new synapse formation/maintenance. Moreover, presence of new but functionally silent synapses enables more robust LTP to occur through rapid conversion of silent synapses to active synapses, resulting in a stronger input-specific modulation of synapses following prolonged network silencing.
Figures




References
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. - PubMed
-
- Bear MF, Rittenhouse CD. Molecular basis for induction of ocular dominance plasticity. J Neurobiol. 1999;41:83–91. - PubMed
-
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. - PubMed
-
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
