Complex movement topography and extrinsic space representation in the rat forelimb motor cortex as defined by long-duration intracortical microstimulation
- PMID: 23365246
- PMCID: PMC6619127
- DOI: 10.1523/JNEUROSCI.3454-12.2013
Complex movement topography and extrinsic space representation in the rat forelimb motor cortex as defined by long-duration intracortical microstimulation
Abstract
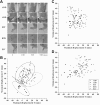
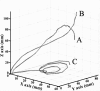
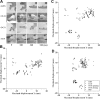
Electrical stimulation of the motor cortex in the rat can evoke complex forelimb multi-joint movements, including movement of limb and paw. In this study, these movements have been quantified in terms of 3D displacement and kinematic variables of two markers positioned on the wrist and middle digits (limb and paw movement, respectively). Electrical microstimulation was applied to the motor cortex using a pulse train of 500 ms duration. Movements were measured using a high-resolution 3D optical system. Five classes of limb movements (abduction, adduction, extension, retraction, elevation) and four classes of paw movements (opening, closure, opening/closure sequence, supination) were described according to their kinematics. A consistent topography of these classes of movements was presented across the motor cortex together with a topography of spatial locations to which the paw was directed. In about one-half of cortical sites, a specific pattern of limb-paw movement combination did exist. Four categories of limb-paw movements resembling behavioral repertoire were identified: reach-shaping, reach-grasp sequence, bring-to-body, and hold-like movement. Overall, the forelimb motor region included: (1) a large caudal forelimb area dominated by reach-shaping movement representation; (2) a small rostral area containing reach-grasp sequence and bring-to-body movement representation; and (3) a more lateral portion where hold-like movement was represented. These results support the view that, in rats, the motor cortex controls forelimb movements at a relatively complex level and suggest that the orderly representation of complex movements and their dynamics/kinematics emerge from the principles of forelimb motor cortex organization.
Figures

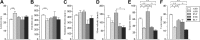




References
-
- Adamovich SV, Archambault PS, Ghafouri M, Levin MF, Poizner H, Feldman AG. Hand trajectory invariance in reaching movements involving the trunk. Exp Brain Res. 2001;138:288–303. - PubMed
-
- Archambault P, Pigeon P, Feldman AG, Levin MF. Recruitment and sequencing of different degrees of freedom during pointing movements involving the trunk in healthy and hemiparetic subjects. Exp Brain Res. 1999;126:55–67. - PubMed
-
- Asanuma H, Arnold A, Zarzecki P. Further study on the excitation of pyramidal tract cells by intracortical microstimulation. Exp Brain Res. 1976;26:443–461. - PubMed
-
- Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res. 1997;84:81–97. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
