Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer's disease
- PMID: 23365250
- PMCID: PMC6571077
- DOI: 10.1523/JNEUROSCI.4437-12.2013
Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer's disease
Abstract
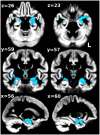
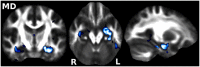
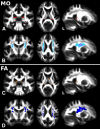
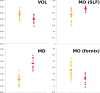
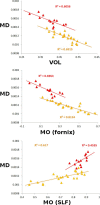
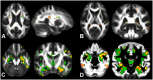
Diffusion imaging is a promising marker of microstructural damage in neurodegenerative disorders, but interpretation of its relationship with underlying neuropathology can be complex. Here, we examined both volumetric and brain microstructure abnormalities in 13 amnestic patients with mild cognitive impairment (MCI), who progressed to probable Alzheimer's disease (AD) no earlier than 2 years after baseline scanning, in order to focus on early, and hence more sensitive, imaging markers. We compared them to 22 stable amnestic MCI patients with similar cognitive performance and episodic memory impairment but who did not show progression of symptoms for at least 3 years. Significant group differences were mainly found in the volume and microstructure of the left hippocampus, while white matter group differences were also found in the body of the fornix, left fimbria, and superior longitudinal fasciculus (SLF). Diffusion index abnormalities in the SLF were the sign of a subtle microstructural injury not detected by standard atrophy measures in the corresponding gray matter regions. The microstructural measure obtained in the left hippocampus using diffusion imaging showed the most substantial differences between the two groups and was the best single predictor of future progression to AD. An optimal prediction model (91% accuracy, 85% sensitivity, 96% specificity) was obtained by combining MRI measures and CSF protein biomarkers. These results highlight the benefit of using the information of brain microstructural damage, in addition to traditional gray matter volume, to detect early, subtle abnormalities in MCI prior to clinical progression to probable AD and, in combination with CSF markers, to accurately predict such progression.
Figures
References
-
- Agosta F, Pievani M, Sala S, Geroldi C, Galluzzi S, Frisoni GB, Filippi M. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology. 2011;258:853–863. - PubMed
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994.
-
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. - PubMed
-
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
