Notum homolog plays a novel role in primary motor innervation
- PMID: 23365253
- PMCID: PMC3711564
- DOI: 10.1523/JNEUROSCI.3694-12.2013
Notum homolog plays a novel role in primary motor innervation
Abstract
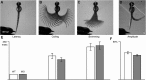
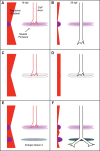
To form complex neuronal networks, growth cones use intermediate targets as guideposts on the path to more distant targets. In the developing zebrafish (Danio rerio), the muscle pioneers (MPs) are intermediate targets for primary motor neurons (PMNs) that innervate the trunk musculature. The mechanisms regulating PMN axon guidance at the MPs are not fully understood. We have identified a new member of the Notum family in zebrafish, Notum 2, which is expressed exclusively in the MPs during primary motor innervation. While homologs of Notum, including zebrafish Notum 1a, negatively regulate the Wnt/β-catenin signaling pathway, we discovered a novel function of Notum 2 in regulating motor axon guidance. Knockdown of Notum 2 resulted in a failure of caudal primary (CaP) axons to migrate beyond the MPs, despite the proper specification of the intermediate target. In contrast, mosaic Notum 2 overexpression induced branching of PMN axons. This effect is specific to Notum 2, as overexpression of Notum 1a does not affect PMN axon trajectory. Ectopic expression of Notum 2 by cells contacting the growing CaP axon induced the highest frequency of branching, suggesting that localized Notum 2 expression affects axon behavior. We propose a model where Notum 2 expression at the MPs provides a cue to release CaP motor axons from their intermediate targets, allowing growth cones to proceed to secondary targets in the ventral muscle. This work demonstrates an unexpected role for a Notum homolog in regulating growth cone migration, separate from the well established functions of other Notum homologs in Wnt signaling.
Figures

References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Ayers KL, Gallet A, Staccini-Lavenant L, Thérond PP. The long-range activity of Hedgehog is regulated in the apical extracellular space by the glypican Dally and the hydrolase Notum. Dev Cell. 2010;18:605–620. - PubMed
-
- Beckett K, Franch-Marro X, Vincent JP. Glypican-mediated endocytosis of Hedgehog has opposite effects in flies and mice. Trends Cell Biol. 2008;18:360–363. - PubMed
-
- Benowitz LI, Yin Y. Combinatorial treatments for promoting axon regeneration in the CNS: strategies for overcoming inhibitory signals and activating neurons' intrinsic growth state. Dev Neurobiol. 2007;67:1148–1165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
