An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in Guinea pigs
- PMID: 23365421
- PMCID: PMC3624190
- DOI: 10.1128/JVI.02745-12
An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in Guinea pigs
Abstract
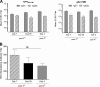
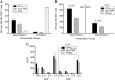
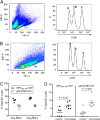
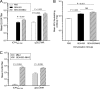
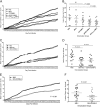
Immunotherapeutic herpes simplex virus 2 (HSV-2) vaccine efficacy depends upon the promotion of antigen-specific immune responses that inhibit reactivation or reactivated virus, thus controlling both recurrent lesions and viral shedding. In the present study, a candidate subunit vaccine, GEN-003/MM-2, was evaluated for its ability to induce a broad-spectrum immune response in mice and therapeutic efficacy in HSV-2-infected guinea pigs. GEN-003 is comprised of HSV-2 glycoprotein D2 (gD2ΔTMR340-363) and a truncated form of infected cell polypeptide 4 (ICP4383-766), formulated with Matrix M-2 (MM-2) adjuvant (GEN-003/MM-2). In addition to eliciting humoral immune responses, CD4(+) and CD8(+) T cells characterized by the secretion of multiple cytokines and cytolytic antigen-specific T cell responses that were able to be recalled at least 44 days after the last immunization were induced in immunized mice. Furthermore, vaccination with either GEN-003 or GEN-003/MM-2 led to significant reductions in both the prevalence and severity of lesions in HSV-2-infected guinea pigs compared to those of phosphate-buffered saline (PBS) control-vaccinated animals. While vaccination with MM-2 adjuvant alone decreased recurrent disease symptoms compared to the PBS control group, the difference was not statistically significant. Importantly, the frequency of recurrent viral shedding was considerably reduced in GEN-003/MM-2-vaccinated animals but not in GEN-003- or MM-2-vaccinated animals. These findings suggest a possible role for immunotherapeutic GEN-003/MM-2 vaccination as a viable alternative to chronic antiviral drugs in the treatment and control of genital herpes disease.
Figures

References
-
- Muggeridge MI, Robert SR, Isola VJ, Cohen GH, Eisenberg RJ. (ed). 1990. Herpes simplex virus glycoproteins. Elsevier, New York, NY
-
- Corey L. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331–340 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

