Release of severe acute respiratory syndrome coronavirus nuclear import block enhances host transcription in human lung cells
- PMID: 23365422
- PMCID: PMC3624188
- DOI: 10.1128/JVI.02520-12
Release of severe acute respiratory syndrome coronavirus nuclear import block enhances host transcription in human lung cells
Abstract
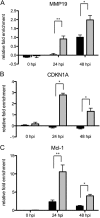
The severe acute respiratory syndrome coronavirus accessory protein ORF6 antagonizes interferon signaling by blocking karyopherin-mediated nuclear import processes. Viral nuclear import antagonists, expressed by several highly pathogenic RNA viruses, likely mediate pleiotropic effects on host gene expression, presumably interfering with transcription factors, cytokines, hormones, and/or signaling cascades that occur in response to infection. By bioinformatic and systems biology approaches, we evaluated the impact of nuclear import antagonism on host expression networks by using human lung epithelial cells infected with either wild-type virus or a mutant that does not express ORF6 protein. Microarray analysis revealed significant changes in differential gene expression, with approximately twice as many upregulated genes in the mutant virus samples by 48 h postinfection, despite identical viral titers. Our data demonstrated that ORF6 protein expression attenuates the activity of numerous karyopherin-dependent host transcription factors (VDR, CREB1, SMAD4, p53, EpasI, and Oct3/4) that are critical for establishing antiviral responses and regulating key host responses during virus infection. Results were confirmed by proteomic and chromatin immunoprecipitation assay analyses and in parallel microarray studies using infected primary human airway epithelial cell cultures. The data strongly support the hypothesis that viral antagonists of nuclear import actively manipulate host responses in specific hierarchical patterns, contributing to the viral pathogenic potential in vivo. Importantly, these studies and modeling approaches not only provide templates for evaluating virus antagonism of nuclear import processes but also can reveal candidate cellular genes and pathways that may significantly influence disease outcomes following severe acute respiratory syndrome coronavirus infection in vivo.
Figures


References
-
- Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967–1976 - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953–1966 - PubMed
-
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, Butt KM, Wong KL, Chan KW, Lim W, Shortridge KF, Yuen KY, Peiris JS, Poon LL. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276–278 - PubMed
-
- Lau SK, Woo PC, Li KS, Huang Y, Wang M, Lam CS, Xu H, Guo R, Chan KH, Zheng BJ, Yuen KY. 2007. Complete genome sequence of bat coronavirus HKU2 from Chinese horseshoe bats revealed a much smaller spike gene with a different evolutionary lineage from the rest of the genome. Virology 367:428–439 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

