Antigenicity and immunogenicity of transmitted/founder, consensus, and chronic envelope glycoproteins of human immunodeficiency virus type 1
- PMID: 23365441
- PMCID: PMC3624376
- DOI: 10.1128/JVI.02297-12
Antigenicity and immunogenicity of transmitted/founder, consensus, and chronic envelope glycoproteins of human immunodeficiency virus type 1
Abstract
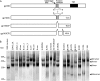
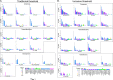
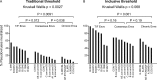
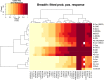
Human immunodeficiency virus type 1 (HIV-1) vaccine development requires selection of appropriate envelope (Env) immunogens. Twenty HIV-1 Env glycoproteins were examined for their ability to bind human anti-HIV-1 monoclonal antibodies (MAbs) and then used as immunogens in guinea pigs to identify promising immunogens. These included five Envs derived from chronically infected individuals, each representing one of five common clades and eight consensus Envs based on these five clades, as well as the consensus of the entire HIV-1 M group, and seven transmitted/founder (T/F) Envs from clades B and C. Sera from immunized guinea pigs were tested for neutralizing activity using 36 HIV-1 Env-pseudotyped viruses. All Envs bound to CD4 binding site, membrane-proximal, and V1/V2 MAbs with similar apparent affinities, although the T/F Envs bound with higher affinity to the MAb 17b, a CCR5 coreceptor binding site antibody. However, the various Envs differed in their ability to induce neutralizing antibodies. Consensus Envs elicited the most potent responses, but neutralized only a subset of viruses, including mostly easy-to-neutralize tier 1 and some more-difficult-to-neutralize tier 2 viruses. T/F Envs elicited fewer potent neutralizing antibodies but exhibited greater breadth than chronic or consensus Envs. Finally, chronic Envs elicited the lowest level and most limited breadth of neutralizing antibodies overall. Thus, each group of Env immunogens elicited a different antibody response profile. The complementary benefits of consensus and T/F Env immunogens raise the possibility that vaccines utilizing a combination of consensus and T/F Envs may be able to induce neutralizing responses with greater breadth and potency than single Env immunogens.
Figures



References
-
- Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, Novitsky V, Haynes B, Hahn BH, Bhattacharya T, Korber B. 2002. Diversity considerations in HIV-1 vaccine selection. Science 296:2354–2360 - PubMed
-
- Fischer W, Perkins S, Theiler J, Bhattacharya T, Yusim K, Funkhouser R, Kuiken C, Haynes B, Letvin NL, Walker BD, Hahn BH, Korber BT. 2007. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat. Med. 13:100–106 - PubMed
-
- Letourneau S, Im EJ, Mashishi T, Brereton C, Bridgeman A, Yang H, Dorrell L, Dong T, Korber B, McMichael AJ, Hanke T. 2007. Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One 2:e984 doi:10.1371/journal.pone.0000984 - DOI - PMC - PubMed
-
- Rosario M, Borthwick N, Stewart-Jones GB, Mbewe-Mvula A, Bridgeman A, Colloca S, Montefiori D, McMichael AJ, Nicosia A, Quakkelaar ED, Drijfhout JW, Melief CJ, Hanke T. 2012. Prime-boost regimens with adjuvanted synthetic long peptides elicit T cells and antibodies to conserved regions of HIV-1 in macaques. AIDS 26:275–284 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

