Differential control of Helios(+/-) Treg development by monocyte subsets through disparate inflammatory cytokines
- PMID: 23365462
- PMCID: PMC3612859
- DOI: 10.1182/blood-2012-11-469122
Differential control of Helios(+/-) Treg development by monocyte subsets through disparate inflammatory cytokines
Abstract
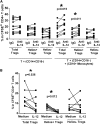
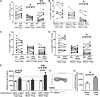
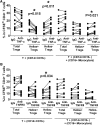
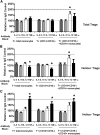
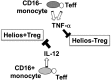
Foxp3(+) regulatory T cells (Tregs) play a pivotal role in control of autoimmunity and pathological immune responses. Helios, the Ikarus family transcription factor, binds to the Foxp3 promoter, stabilizing its expression, and is expressed in 70% of peripheral Tregs of healthy individuals. This frequency is altered during malignancy, infection, and autoimmunity, although the mechanisms that control proliferation and relative numbers of Helios(+/-) Tregs remain largely unknown. Using a T-cell-monocyte in vitro stimulation assay, we now show that proliferation of Helios(+) Tregs is inhibited by CD16(+) monocyte subset. Antibody blocking with anti-interleukin (IL)-12 reversed this inhibition, whereas addition of IL-12 suppressed Helios(+) Treg expansion, indicating that CD16(+) monocyte control of Helios(+) Treg numbers is mediated through IL-12. In contrast, proliferation of Helios(-) Tregs, which express higher levels of tumor necrosis factor receptor II (TNFRII), was suppressed by TNF-α, whereas anti-TNF-α and anti-TNFRII reversed the inhibition. CD16(-) monocyte subset was mainly responsible for TNF-α-mediated control of Helios(-) Treg expansion. Altogether, these data suggest a differential role for monocyte subsets in control of Helios(+/-) Treg development that is mediated by distinct inflammatory cytokines. These data may have important implications for understanding the pathogenesis as well as control of chronic inflammatory and autoimmune diseases.
Figures

Similar articles
-
Differences in Maturation Status and Immune Phenotypes of Circulating Helios+ and Helios- Tregs and Their Disrupted Correlations With Monocyte Subsets in Autoantibody-Positive T1D Individuals.Front Immunol. 2021 May 12;12:628504. doi: 10.3389/fimmu.2021.628504. eCollection 2021. Front Immunol. 2021. PMID: 34054801 Free PMC article.
-
Foxp3+Helios+ regulatory T cells are associated with monocyte subsets and their PD-1 expression during acute HIV-1 infection.BMC Immunol. 2019 Oct 24;20(1):38. doi: 10.1186/s12865-019-0319-7. BMC Immunol. 2019. PMID: 31651258 Free PMC article.
-
IL-1R1 is expressed on both Helios(+) and Helios(-) FoxP3(+) CD4(+) T cells in the rheumatic joint.Clin Exp Immunol. 2015 Oct;182(1):90-100. doi: 10.1111/cei.12668. Epub 2015 Jul 30. Clin Exp Immunol. 2015. PMID: 26076982 Free PMC article.
-
Coexpression of Helios in Foxp3+ Regulatory T Cells and Its Role in Human Disease.Dis Markers. 2021 Jun 22;2021:5574472. doi: 10.1155/2021/5574472. eCollection 2021. Dis Markers. 2021. PMID: 34257746 Free PMC article. Review.
-
Helios: still behind the clouds.Immunology. 2019 Nov;158(3):161-170. doi: 10.1111/imm.13115. Epub 2019 Oct 13. Immunology. 2019. PMID: 31517385 Free PMC article. Review.
Cited by
-
Hemin controls T cell polarization in sickle cell alloimmunization.J Immunol. 2014 Jul 1;193(1):102-10. doi: 10.4049/jimmunol.1400105. Epub 2014 May 30. J Immunol. 2014. PMID: 24879794 Free PMC article. Clinical Trial.
-
The Interplay Between Monocytes/Macrophages and CD4(+) T Cell Subsets in Rheumatoid Arthritis.Front Immunol. 2015 Nov 19;6:571. doi: 10.3389/fimmu.2015.00571. eCollection 2015. Front Immunol. 2015. PMID: 26635790 Free PMC article. Review.
-
A Praziquantel Treatment Study of Immune and Transcriptome Profiles in Schistosoma haematobium-Infected Gabonese Schoolchildren.J Infect Dis. 2020 Nov 13;222(12):2103-2113. doi: 10.1093/infdis/jiz641. J Infect Dis. 2020. PMID: 31844885 Free PMC article.
-
A Phase II Clinical Trial of TRC105 (Anti-Endoglin Antibody) in Adults With Advanced/Metastatic Urothelial Carcinoma.Clin Genitourin Cancer. 2017 Feb;15(1):77-85. doi: 10.1016/j.clgc.2016.05.010. Epub 2016 May 27. Clin Genitourin Cancer. 2017. PMID: 27328856 Free PMC article. Clinical Trial.
-
Immunological Changes in Monocyte Subsets and Their Association With Foxp3+ Regulatory T Cells in HIV-1-Infected Individuals With Syphilis: A Brief Research Report.Front Immunol. 2019 Apr 9;10:714. doi: 10.3389/fimmu.2019.00714. eCollection 2019. Front Immunol. 2019. PMID: 31024549 Free PMC article.
References
-
- Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

