Exhaled breath condensate: a promising source for biomarkers of lung disease
- PMID: 23365513
- PMCID: PMC3539342
- DOI: 10.1100/2012/217518
Exhaled breath condensate: a promising source for biomarkers of lung disease
Abstract
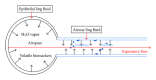
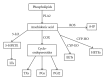
Exhaled breath condensate (EBC) has been increasingly studied as a noninvasive research method for sampling the alveolar and airway space and is recognized as a promising source of biomarkers of lung diseases. Substances measured in EBC include oxidative stress and inflammatory mediators, such as arachidonic acid derivatives, reactive oxygen/nitrogen species, reduced and oxidized glutathione, and inflammatory cytokines. Although EBC has great potential as a source of biomarkers in many lung diseases, the low concentrations of compounds within the EBC present challenges in sample collection and analysis. Although EBC is viewed as a noninvasive method for sampling airway lining fluid (ALF), validation is necessary to confirm that EBC truly represents the ALF. Likewise, a dilution factor for the EBC is needed in order to compare across subjects and determine changes in the ALF. The aims of this paper are to address the characteristics of EBC; strategies to standardize EBC sample collection and review available analytical techniques for EBC analysis.
Figures
Similar articles
-
Exhaled breath condensate: determination of non-volatile compounds and their potential for clinical diagnosis and monitoring. A review.Anal Chim Acta. 2013 Dec 17;805:1-18. doi: 10.1016/j.aca.2013.07.049. Epub 2013 Jul 31. Anal Chim Acta. 2013. PMID: 24296139 Review.
-
Exhaled Breath Condensate: An Update.Immunol Allergy Clin North Am. 2018 Nov;38(4):667-678. doi: 10.1016/j.iac.2018.06.002. Epub 2018 Sep 21. Immunol Allergy Clin North Am. 2018. PMID: 30342587 Review.
-
Exhaled breath condensate: an overview.Immunol Allergy Clin North Am. 2007 Nov;27(4):587-96; v. doi: 10.1016/j.iac.2007.09.001. Immunol Allergy Clin North Am. 2007. PMID: 17996577 Free PMC article. Review.
-
Comparison of biomarkers in exhaled breath condensate and bronchoalveolar lavage.Am J Respir Crit Care Med. 2007 Feb 1;175(3):222-7. doi: 10.1164/rccm.200601-107OC. Epub 2006 Nov 16. Am J Respir Crit Care Med. 2007. PMID: 17110649
-
Non-invasive collection of exhaled breath condensate in rats: evaluation of pH, H₂O₂ and NOx in lipopolysaccharide-induced acute lung injury.Vet J. 2012 Nov;194(2):222-8. doi: 10.1016/j.tvjl.2012.04.009. Epub 2012 Jun 1. Vet J. 2012. PMID: 22658821
Cited by
-
Utilizing nitrogen, sulfur, phosphorus, and chlorine co-doped carbon dots as a fluorescent probe for determination of vancomycin in exhaled breath condensate.Heliyon. 2024 Aug 30;10(17):e37253. doi: 10.1016/j.heliyon.2024.e37253. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39286091 Free PMC article.
-
Quantification of malondialdehyde in exhaled breath condensate using pseudo two-dimensional ultra-performance liquid chromatography coupled with single quadrupole mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Jan 15;1105:210-216. doi: 10.1016/j.jchromb.2018.12.009. Epub 2018 Dec 13. J Chromatogr B Analyt Technol Biomed Life Sci. 2019. PMID: 30611078 Free PMC article.
-
Breathprints of model murine bacterial lung infections are linked with immune response.Eur Respir J. 2015 Jan;45(1):181-90. doi: 10.1183/09031936.00015814. Epub 2014 Oct 16. Eur Respir J. 2015. PMID: 25323243 Free PMC article.
-
Biomarkers in Exhaled Breath Condensate and Serum of Chronic Obstructive Pulmonary Disease and Non-Small-Cell Lung Cancer.Int J Chronic Dis. 2013;2013:578613. doi: 10.1155/2013/578613. Epub 2013 Aug 1. Int J Chronic Dis. 2013. PMID: 26464846 Free PMC article. Review.
-
Specific Metabolome Profile of Exhaled Breath Condensate in Patients with Shock and Respiratory Failure: A Pilot Study.Metabolites. 2016 Sep 1;6(3):26. doi: 10.3390/metabo6030026. Metabolites. 2016. PMID: 27598216 Free PMC article.
References
-
- Horváth I, Hunt J, Barnes PJ, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. European Respiratory Journal. 2005;26(3):523–548. - PubMed
-
- Montuschi P. Analysis of exhaled breath condensate in respiratory medicine: methodological aspects and potential clinical applications. Therapeutic Advances in Respiratory Disease. 2007;1(1):5–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

