Impact of GLP-1 receptor agonists on major gastrointestinal disorders for type 2 diabetes mellitus: a mixed treatment comparison meta-analysis
- PMID: 23365557
- PMCID: PMC3540917
- DOI: 10.1155/2012/230624
Impact of GLP-1 receptor agonists on major gastrointestinal disorders for type 2 diabetes mellitus: a mixed treatment comparison meta-analysis
Abstract
Aim: We aimed to integrate evidence from all randomized controlled trials (RCTs) and assess the impact of different doses of exenatide or liraglutide on major gastrointestinal adverse events (GIAEs) in type 2 diabetes (T2DM).
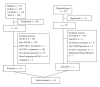
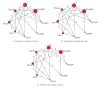
Methods: RCTs evaluating different doses of exenatide and liraglutide against placebo or an active comparator with treatment duration ≥4 weeks were searched and reviewed. A total of 35, 32 and 28 RCTs met the selection criteria evaluated for nausea, vomiting, and diarrhea, respectively. Pairwise random-effects meta-analyses and mixed treatment comparisons (MTC) of all RCTs were performed.
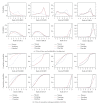
Results: All GLP-1 dose groups significantly increased the probability of nausea, vomiting and diarrhea relative to placebo and conventional treatment. MTC meta-analysis showed that there was 99.2% and 85.0% probability, respectively, that people with exenatide 10 μg twice daily (EX10BID) was more vulnerable to nausea and vomiting than those with other treatments. There was a 78.90% probability that liraglutide 1.2 mg once daily (LIR1.2) has a higher risk of diarrhea than other groups. A dose-dependent relationship of exenatide and liraglutide on GIAEs was observed.
Conclusions: Our MTC meta-analysis suggests that patients should be warned about these GIAEs in early stage of treatment by GLP-1s, especially by EX10BID and LIR1.2, to promote treatment compliance.
Figures
Similar articles
-
Effects of glucagon-like peptide-1 receptor agonists on weight loss in patients with type 2 diabetes: a systematic review and network meta-analysis.J Diabetes Res. 2015;2015:157201. doi: 10.1155/2015/157201. Epub 2015 Jan 20. J Diabetes Res. 2015. PMID: 25688373 Free PMC article.
-
GLP-1 receptor agonists and HBA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials.Curr Med Res Opin. 2011 Aug;27(8):1519-28. doi: 10.1185/03007995.2011.590127. Epub 2011 Jun 13. Curr Med Res Opin. 2011. PMID: 21663496
-
Comparison of safety and tolerability with continuous (exenatide once weekly) or intermittent (exenatide twice daily) GLP-1 receptor agonism in patients with type 2 diabetes.Diabetes Obes Metab. 2012 Dec;14(12):1097-103. doi: 10.1111/j.1463-1326.2012.01639.x. Epub 2012 Jul 19. Diabetes Obes Metab. 2012. PMID: 22734440 Free PMC article.
-
Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: A systematic analysis of published clinical trials.Diabetes Obes Metab. 2017 Mar;19(3):336-347. doi: 10.1111/dom.12824. Epub 2016 Dec 19. Diabetes Obes Metab. 2017. PMID: 27860132 Review.
-
[Twice-daily and weekly exenatide: clinical profile of two pioneer formulations in incretin therapy].Med Clin (Barc). 2014 Sep;143 Suppl 2:23-7. doi: 10.1016/S0025-7753(14)70105-8. Epub 2014 Oct 15. Med Clin (Barc). 2014. PMID: 25437462 Review. Spanish.
Cited by
-
Optimal dose of tirzepatide for type 2 diabetes mellitus: A meta-analysis and trial sequential analysis.Front Cardiovasc Med. 2022 Aug 31;9:990182. doi: 10.3389/fcvm.2022.990182. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36119737 Free PMC article.
-
TLQP-21 protects human umbilical vein endothelial cells against high-glucose-induced apoptosis by increasing G6PD expression.PLoS One. 2013 Nov 21;8(11):e79760. doi: 10.1371/journal.pone.0079760. eCollection 2013. PLoS One. 2013. PMID: 24278172 Free PMC article.
-
Recent Advances and Therapeutic Benefits of Glucagon-Like Peptide-1 (GLP-1) Agonists in the Management of Type 2 Diabetes and Associated Metabolic Disorders.Cureus. 2024 Oct 21;16(10):e72080. doi: 10.7759/cureus.72080. eCollection 2024 Oct. Cureus. 2024. PMID: 39574978 Free PMC article. Review.
-
Glucagon-Like Peptide-1 Receptor Analogues in Type 2 Diabetes: Their Use and Differential Features.Clin Drug Investig. 2019 Aug;39(8):805-819. doi: 10.1007/s40261-019-00826-0. Clin Drug Investig. 2019. PMID: 31317516 Free PMC article. Review.
-
Efficacy and safety of dulaglutide in patients with type 2 diabetes: a meta-analysis and systematic review.Sci Rep. 2016 Jan 8;6:18904. doi: 10.1038/srep18904. Sci Rep. 2016. PMID: 26742577 Free PMC article.
References
-
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. - PubMed
-
- Verspohl EJ. Novel therapeutics for type 2 diabetes: incretin hormone mimetics (glucagon-like peptide-1 receptor agonists) and dipeptidyl peptidase-4 inhibitors. Pharmacology and Therapeutics. 2009;124(1):113–138. - PubMed
-
- Hansen KB, Knop FK, Holst JJ, Vilsbøll T. Treatment of type 2 diabetes with glucagon-like peptide-1 receptor agonists. International Journal of Clinical Practice. 2009;63(8):1154–1160. - PubMed
-
- Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359(9309):824–830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
