Baculovirus superinfection: a probable restriction factor on the surface display of proteins for library screening
- PMID: 23365677
- PMCID: PMC3554712
- DOI: 10.1371/journal.pone.0054631
Baculovirus superinfection: a probable restriction factor on the surface display of proteins for library screening
Abstract
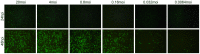
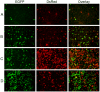
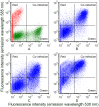
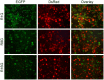
In addition to the expression of recombinant proteins, baculoviruses have been developed as a platform for the display of complex eukaryotic proteins on the surface of virus particles or infected insect cells. Surface display has been used extensively for antigen presentation and targeted gene delivery but is also a candidate for the display of protein libraries for molecular screening. However, although baculovirus gene libraries can be efficiently expressed and displayed on the surface of insect cells, target gene selection is inefficient probably due to super-infection which gives rise to cells expressing more than one protein. In this report baculovirus superinfection of Sf9 cells has been investigated by the use of two recombinant multiple nucleopolyhedrovirus carrying green or red fluorescent proteins under the control of both early and late promoters (vAcBacGFP and vAcBacDsRed). The reporter gene expression was detected 8 hours after the infection of vAcBacGFP and cells in early and late phases of infection could be distinguished by the fluorescence intensity of the expressed protein. Simultaneous infection with vAcBacGFP and vAcBacDsRed viruses each at 0.5 MOI resulted in 80% of infected cells co-expressing the two fluorescent proteins at 48 hours post infection (hpi), and subsequent infection with the two viruses resulted in similar co-infection rate. Most Sf9 cells were re-infectable within the first several hours post infection, but the re-infection rate then decreased to a very low level by 16 hpi. Our data demonstrate that Sf9 cells were easily super-infectable during baculovirus infection, and super-infection could occur simultaneously at the time of the primary infection or subsequently during secondary infection by progeny viruses. The efficiency of super-infection may explain the difficulties of baculovirus display library screening but would benefit the production of complex proteins requiring co-expression of multiple polypeptides.
Conflict of interest statement
Figures




References
-
- Hitchman RB, Possee RD, King LA (2009) Baculovirus Expression Systems for Recombinant Protein Production in Insect Cells. Recent Patents on Biotechnology 3: 46–54. - PubMed
-
- Makela AR, Oker-Blom C (2008) The Baculovirus Display Technology-An Evolving Instrument for Molecular Screening and Drug Delivery. Combinatorial Chemistry & High Throughput Screening 11: 86–98. - PubMed
-
- James DC, Freedman RB, Hoare M, Ogonah OW, Rooney BC, et al. (1995) N-Glycosylation of recombinant human interferon-gamma produced in different animal expression systems. Biotechnology (NY) 13: 592–596. - PubMed
-
- Hodder AN, Crewther PE, Matthew ML, Reid GE, Moritz RL, et al. (1996) The disulfide bond structure of Plasmodium apical membrane antigen-1. J Biol Chem 271: 29446–29452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

