Profiling of humoral response to influenza A(H1N1)pdm09 infection and vaccination measured by a protein microarray in persons with and without history of seasonal vaccination
- PMID: 23365683
- PMCID: PMC3554683
- DOI: 10.1371/journal.pone.0054890
Profiling of humoral response to influenza A(H1N1)pdm09 infection and vaccination measured by a protein microarray in persons with and without history of seasonal vaccination
Abstract
Background: The influence of prior seasonal influenza vaccination on the antibody response produced by natural infection or vaccination is not well understood.
Methods: We compared the profiles of antibody responses of 32 naturally infected subjects and 98 subjects vaccinated with a 2009 influenza A(H1N1) monovalent MF59-adjuvanted vaccine (Focetria, Novartis), with and without a history of seasonal influenza vaccination. Antibodies were measured by hemagglutination inhibition (HI) assay for influenza A(H1N1)pdm09 and by protein microarray (PA) using the HA1 subunit for seven recent and historic H1, H2 and H3 influenza viruses, and three avian influenza viruses. Serum samples for the infection group were taken at the moment of collection of the diagnostic sample, 10 days and 30 days after onset of influenza symptoms. For the vaccination group, samples were drawn at baseline, 3 weeks after the first vaccination and 5 weeks after the second vaccination.
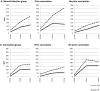
Results: We showed that subjects with a history of seasonal vaccination generally exhibited higher baseline titers for the various HA1 antigens than subjects without a seasonal vaccination history. Infection and pandemic influenza vaccination responses in persons with a history of seasonal vaccination were skewed towards historic antigens.
Conclusions: Seasonal vaccination is of significant influence on the antibody response to subsequent infection and vaccination, and further research is needed to understand the effect of annual vaccination on protective immunity.
Conflict of interest statement
Figures

References
-
- WHO (2011) Available: http://www.euro.who.int/influenza/ah1n1.Accessed 2012 Jun 28.
-
- Jia N, de Vlas SJ, Liu YX, Zhang JS, Zhan L, et al. (2009) Serological reports of human infections of H7 and H9 avian influenza viruses in northern China. J Clin Virol 44: 225–229. - PubMed
-
- Russell RJ, Burns WH, White DO, Anders EM, Ward CW, et al. (1979) Antigenic determinants of influenza virus hemagglutinin. III. Competitive binding of antibodies directed against “common” and “strain-specific” antigenic determinants of A/Memphis/72 hemagglutinin. J Immunol 123: 825–832. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

