Reinforcement mechanisms in putamen during high frequency STN DBS: A point process study
- PMID: 23366116
- PMCID: PMC3822770
- DOI: 10.1109/EMBC.2012.6346155
Reinforcement mechanisms in putamen during high frequency STN DBS: A point process study
Abstract
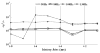
Despite a pivotal role in the motor loop, dorsolateral striatum (putamen) has been poorly studied thus far under Parkinsonian conditions and Deep Brain Stimulation (DBS). We analyze the activity of the putamen in a monkey by combining single unit recordings and point process models. The animal received DBS (30-130 Hz) in the subthalamic nucleus (STN) while at rest and recordings were acquired both before and after treatment with 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP), which induced Parkinsonian-like motor disorders. 141 neurons were collected and, for each neuron, a point process model captured DBS-evoked discharge patterns. In the normal animal, spike trains at rest had Poisson like distribution with non-stationary recurrent patterns (RPs) of period 3-7 ms and were mildly changed by low frequency (LF, i.e., < 100 Hz) DBS (i.e., < 20% of neurons affected). With high frequency (HF, i.e., 100-130 Hz) DBS, instead, up to 59% of neurons were affected, the DBS history significantly impacted the neuronal spiking propensity, and the RPs and the post-stimulus activation latency decreased. MPTP evoked inter-neuronal dependencies (INDs) at rest and, compared to normal, LF DBS of the MPTP animal increased RPs and INDs, while HF DBS elicited a faster and wider post-stimulus activation. Overall, HF DBS reduced ongoing non-stationary dynamics by regularizing the discharge patterns both in MPTP and normal putamen, while the combination of MPTP and LF DBS enhanced such dynamics.
Figures
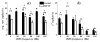

References
-
- Koller W, Pahwa R, Busenbark K, et al. High-frequency unilateral thalamic stimulation in the treatment of essential and Parkinsonian tremor. Ann Neurol. 1997;42:292–299. - PubMed
-
- Vitek JL, Hashimoto T, Peoples J, DeLong MR, Bakay AE. Acute stimulation in the external segment of the globus pallidus improves Parkinsonian motor signs. Mov Disord. 2004;19:907–915. - PubMed
-
- Moro E, Lozano AM, Pollak P, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord. 2010;25:578–586. - PubMed
-
- Montgomery EB, Jr, Gale JT. Mechanisms of action of deep brain stimulation (DBS) Neurosci Biobehav Rev. 2008;32:388–407. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
