The impact of routine cryptococcal antigen screening on survival among HIV-infected individuals with advanced immunosuppression in Kenya
- PMID: 23368667
- PMCID: PMC3809891
- DOI: 10.1111/tmi.12067
The impact of routine cryptococcal antigen screening on survival among HIV-infected individuals with advanced immunosuppression in Kenya
Abstract
Objectives: To test the hypothesis that a screening and treatment intervention for early cryptococcal infection would improve survival among HIV-infected individuals with low CD4 cell counts.
Methods: Newly enrolled patients at Family AIDS Care and Education Services (FACES) in Kenya with CD4 ≤ 100 cells/μl were tested for serum cryptococcal antigen (sCrAg). Individuals with sCrAg titre ≥ 1:2 were treated with high-dose fluconazole. Cox proportional hazard models of Kaplan-Meier curves were used to compare survival among individuals with CD4 ≤ 100 cells/μl in the intervention and historical control groups.
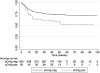
Results: The median age was 34 years [IQR: 29,41], 54% were female, and median CD4 was 43 cells/μl [IQR: 18,71]. Follow-up time was 1224 person-years. In the intervention group, 66% (514/782) were tested for sCrAg; of whom, 11% (59/514) were sCrAg positive. Mortality was 25% (196/782) in the intervention group and 25% (191/771) in the control group. There was no significant difference between the intervention and control group in overall survival [hazard ratio (HR): 1.1 (95%CI:0.9,1.3)] or three-month survival [HR: 1.0 (95%CI:0.8,1.3)]. Within the intervention group, sCrAg-positive individuals had significantly lower survival rates than sCrAg-negative individuals [HR:1.8 (95%CI: 1.0, 3.0)].
Conclusions: A screening and treatment intervention to identify sCrAg-positive individuals and treat them with high-dose fluconazole did not significantly improve overall survival among HIV-infected individuals with CD4 counts ≤ 100 cells/μl compared to a historical control, perhaps due to intervention uptake rates or poor efficacy of high-dose oral fluconazole.
© 2013 Blackwell Publishing Ltd.
Conflict of interest statement
Declarations of Interest: Dr. Meyer has received honoraria from Abbott and a drug donation from Valeant Pharmaceuticals., Ms. Kendi has no conflicts of interest to disclose., Dr. Penner has no conflicts of interest to disclose., Mr. Odhiambo has no conflicts of interest to disclose., Mr. Otieno has no conflicts of interest to disclose., Dr. Bukusi has no conflicts of interest to disclose., Dr. Cohen has no conflicts of interest to disclose
Figures

References
-
- Tansuphasawadikul S, Amornkul PN, Tanchanpong C, Limpakarnjanarat K, Kaewkungwal J, Likanonsakul S, et al. Clinical presentation of hospitalized adult patients with HIV infection and AIDS in Bangkok, Thailand. J Acquir Immune Defic Syndr. 1999 Aug 1;21(4):326–32. - PubMed
-
- Chariyalertsak S, Sirisanthana T, Saengwonloey O, Nelson KE. Clinical presentation and risk behaviors of patients with acquired immunodeficiency syndrome in Thailand, 1994--1998: regional variation and temporal trends. Clin Infect Dis. 2001 Mar 15;32(6):955–62. - PubMed
-
- French N, Gray K, Watera C, Nakiyingi J, Lugada E, Moore M, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002 May 3;16(7):1031–8. - PubMed
-
- Park B, Wannemuehler K, Marston B, Govender N, Pappas P, Chiller T. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

