Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study
- PMID: 23369412
- PMCID: PMC3576519
- DOI: 10.1016/S1473-3099(12)70345-6
Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study
Abstract
Background: Replication-competent virus vector vaccines might have advantages compared with non-replicating vector vaccines. We tested the safety and immunogenicity of an oral adenovirus serotype 4 vector vaccine candidate (Ad4-H5-Vtn) expressing the haemagglutinin from an avian influenza A H5N1 virus.
Methods: We did this phase 1 study at four sites in the USA. We used a computer-generated randomisation list (block size eight, stratified by site) to assign healthy volunteers aged 18-40 years to receive one of five doses of Ad4-H5-Vtn (10(7) viral particles [VP], 10(8) VP, 10(9) VP, 10(10) VP, 10(11) VP) or placebo (3:1). Vaccine or placebo was given on three occasions, about 56 days apart. Participants, investigators, and study-site personnel were masked to assignment throughout the study. Subsequently, volunteers received a boost dose with 90 μg of an inactivated parenteral H5N1 vaccine. Primary immunogenicity endpoints were seroconversion by haemagglutination-inhibition (HAI), defined as a four-times rise compared with baseline titre, and HAI geometric mean titre (GMT). We solicited symptoms of reactogenicity daily for 7 days after each vaccination and recorded symptoms that persisted beyond 7 days as adverse events. Primary analysis was per protocol. This trial is registered with ClinicalTrials.gov, number NCT01006798.
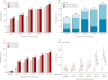
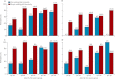
Findings: We enrolled 166 participants (125 vaccine; 41 placebo) between Oct 19, 2009, and Sept 9, 2010. HAI responses were low: 13 of 123 vaccinees (11%, 95% CI 6-17) and three of 41 placebo recipients (7%, 2-20) seroconverted. HAI GMT was 6 (95% CI 5-7) for vaccinees, and 5 (5-6) for placebo recipients. However, when inactivated H5N1 vaccine became available, one H5N1 boost was offered to all participants. In this substudy, HAI seroconversion occurred in 19 of 19 participants in the 10(11) VP cohort (100%; 95% CI 82-100) and eight of 22 placebo recipients (36%; 17-59); 17 of 19 participants in the 10(11) VP cohort (89%; 67-99) achieved seroprotection compared with four of 22 placebo recipients (18%; 5-40); GMT was 135 (89-205) with 10(11) VP, compared with 13 (7-21) with placebo. The cumulative frequency of abdominal pain, diarrhoea, and nasal congestion after all three vaccinations was significantly higher in vaccinees than placebo recipients (21 [16·8%] of 125 vs one [2·4%] of 41, p=0·017; 24 [19·2%] of 125 vs two [4·9%] of 41, p=0·027; 41 [32·8%] of 125 vs six [14·6%] of 41, p=0·028; respectively). No serious treatment-related adverse events occurred.
Interpretation: Oral Ad4 vector priming might enhance the efficacy of poorly immunogenic vaccines such as H5N1.
Funding: Wellcome Trust Foundation, PaxVax.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
No easy route to a pandemic influenza vaccine.Lancet Infect Dis. 2013 Mar;13(3):188-9. doi: 10.1016/S1473-3099(13)70027-6. Epub 2013 Jan 29. Lancet Infect Dis. 2013. PMID: 23369411 No abstract available.
References
-
- Excler JL, Parks CL, Ackland J, Rees H, Gust ID, Koff WC. Replicating viral vectors as HIV vaccines: summary report from the IAVI-sponsored satellite symposium at the AIDS vaccine 2009 conference. Biologicals. 2010;38:511–521. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

