Contribution of uric acid to cancer risk, recurrence, and mortality
- PMID: 23369448
- PMCID: PMC3560981
- DOI: 10.1186/2001-1326-1-16
Contribution of uric acid to cancer risk, recurrence, and mortality
Abstract
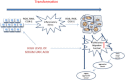
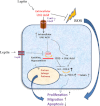
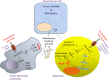
Two risk factors for the development and progression of cancers that are amenable to life style modification are chronic inflammation and the metabolic syndrome. This review proposes two new targets that may mechanistically integrate inflammation and metabolic syndrome, have been largely ignored, and are known to be druggable. Recent evidence has demonstrated that elevated serum uric acid (hyperuricemia) is associated with excess cancer risk, recurrence, and mortality. Although uric acid (UA) can function as a systemic antioxidant, its pro-inflammatory properties have been postulated to play an important role in the pathogenesis of cancer. Furthermore, obesity, Type 2 Diabetes Mellitus (T2DM), and the metabolic syndrome (MetS) are also associated with excess cancer, chronic inflammation, and with hyperuricemia, suggesting that UA may represent an important link between these disorders and the development of cancer. While pharmacological modulation of hyperuricemia could in principal augment anti-cancer therapeutic strategies, some cancer cells express low intracellular levels of the enzyme Xanthine Oxidoreductase (XOR) that are associated with increased cancer aggressiveness and poor clinical outcome. Thus, systemic pharmacological inhibition of XOR may worsen clinical outcome, and specific strategies that target serum uric acid (SUA) without inhibiting tumor cell XOR may create new therapeutic opportunities for cancer associated with hyperuricemia. This review will summarize the evidence that elevated SUA may be a true risk factor for cancer incidence and mortality, and mechanisms by which UA may contribute to cancer pathogenesis will be discussed in the hope that these will identify new opportunities for cancer management.
Figures
References
-
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
