Comparison of 4-chloro-2-nitrophenol adsorption on single-walled and multi-walled carbon nanotubes
- PMID: 23369489
- PMCID: PMC3555131
- DOI: 10.1186/1735-2746-9-5
Comparison of 4-chloro-2-nitrophenol adsorption on single-walled and multi-walled carbon nanotubes
Abstract
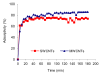
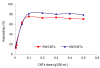
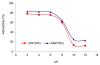
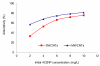
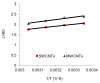
The adsorption characteristics of 4-chloro-2-nitrophenol (4C2NP) onto single-walled and multi-walled carbon nanotubes (SWCNTs and MWCNTs) from aqueous solution were investigated with respect to the changes in the contact time, pH of solution, carbon nanotubes dosage and initial 4C2NP concentration. Experimental results showed that the adsorption efficiency of 4C2NP by carbon nanotubes (both of SWCNTs and MWCNTs) increased with increasing the initial 4C2NP concentration. The maximum adsorption took place in the pH range of 2-6. The linear correlation coefficients of different isotherm models were obtained. Results revealed that the Langmuir isotherm fitted the experimental data better than the others and based on the Langmuir model equation, maximum adsorption capacity of 4C2NP onto SWCNTs and MWCNTs were 1.44 and 4.42 mg/g, respectively. The observed changes in the standard Gibbs free energy, standard enthalpy and standard entropy showed that the adsorption of 4C2NP onto SWCNTs and MWCNTs is spontaneous and exothermic in the temperature range of 298-328 K.
Figures
References
-
- Chen S, Xu ZP, Zhang O, Lu GQ, Hao ZP, Liu S. Studies on adsorption of phenol and 4-nitrophenol on MgAl-mixed oxide derived from MgAl-layered double hydroxide. Sep Purif Technol. 2009;67(2):194–200. doi: 10.1016/j.seppur.2009.03.016. - DOI
-
- Dursun AY, Tepe O. Internal mass transfer effect on biodegradation of phenol by Ca-alginate immobilized Ralstonia eutropha. J Hazard Mater. 2005;126(1–3):105–111. - PubMed
-
- Guidelines for Drinking water Quality: Incorporating First and Second Addenda. 3rd Ed. Vol. 1. World Health Organization, Geneva; 2008. - PubMed
-
- Movahedyan H, Khorsandi H, Salehi R, Nikaeen M. Detection of phenol degrading bacteria and pseudomonas putida in activated sludge by polymerase chain reaction. Iran J Environ Healh Sci Eng. 2009;6(2):115–120.
LinkOut - more resources
Full Text Sources

