Synthesis and application of Amberlite xad-4 functionalized with alizarin red-s for preconcentration and adsorption of rhodium (III)
- PMID: 23369526
- PMCID: PMC3561113
- DOI: 10.1186/1735-2746-9-7
Synthesis and application of Amberlite xad-4 functionalized with alizarin red-s for preconcentration and adsorption of rhodium (III)
Abstract
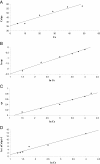
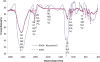
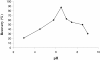
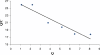
A new chelating resin was prepared by coupling Amberlite XAD-4 with alizarin red-s through an azo spacer, characterized by infra-red spectroscopy and thermal analysis and studied for Rh(III) preconcentration using inductively coupled plasma atomic emission spectroscopy (ICP-AES) for rhodium monitoring in the environment. The optimum pH for sorption of the metal ion was 6.5. The sorption capacity was found 2.1 mg/g of resin for Rh(III). A recovery of 88% was obtained for the metal ion with 1.5 M HCl as eluting agent. Kinetic adsorption data were analyzed by adsorption and desorption times of Rh(III) on modified resin. Scat chard analysis revealed that the homogeneous binding sites were formed in the polymers. The linear regression equation was Q/C = -1.3169Q + 27.222 (R2 = 0.9239), for Rh were formed in the SPE sorbent,Kd and Qmax for the affinity binding sites were calculated to be 0.76 μmol/mL and 20.67 μmol/g, respectively. The equilibrium data and parameters of Rh(III) adsorption on modified resin were analyzed by Langmuir, Freundlich, Temkin and Redlich-Peterson models. The experimental adsorption isotherm was in good concordance with Langmuir and Freundlich models (R2 > 0.998) and based on the Langmuir isotherm the maximum amount of adsorption (qmax) was 4.842 mg/g. The method was applied for rhodium ions determination in environmental samples. with high recovery (>80%).
Figures
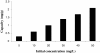



Similar articles
-
New anion-exchange solid-phase extraction support used for preconcentration and determination of lead in water samples by flame atomic absorption spectrometry.J AOAC Int. 2010 Sep-Oct;93(5):1616-24. J AOAC Int. 2010. PMID: 21140675
-
Synthesis, characterization, and application of m-phenylendiamine-modified Amberlite XAD-4 resin for preconcentration and determination of metal ions in water samples.Water Environ Res. 2009 May;81(5):532-9. doi: 10.2175/106143008x370359. Water Environ Res. 2009. PMID: 19472945
-
Grafting of poly[1-(N,N-bis-carboxymethyl)amino-3-allylglycerol-co-dimethylacrylamide] copolymer onto siliceous support for preconcentration and determination of lead (II) in human plasma and environmental samples.J Chromatogr A. 2010 Aug 6;1217(32):5165-72. doi: 10.1016/j.chroma.2010.06.012. Epub 2010 Jun 9. J Chromatogr A. 2010. PMID: 20599201
-
Selective Solid-Phase Extraction of Zinc(II) from Environmental Water Samples Using Ion Imprinted Activated Carbon.J AOAC Int. 2015 Jan-Feb;98(1):206-12. doi: 10.5740/jaoacint.11-293. J AOAC Int. 2015. PMID: 25857899
-
New generation Amberlite XAD resin for the removal of metal ions: A review.J Environ Sci (China). 2015 May 1;31:104-23. doi: 10.1016/j.jes.2014.12.008. Epub 2015 Mar 30. J Environ Sci (China). 2015. PMID: 25968265 Review.
References
-
- Marshall MA, Mottola HA. Synthesis of silica-immobilized 8-quinolinol with (aminophenyl)trimethoxysilane. Anal Chem. 1983;55:2089–2093. doi: 10.1021/ac00263a019. - DOI
-
- Roldan PS, Alcantara IL, Castro GR, Rocha SC, Padilha CCF, Padilha PM. Determination of Cu, Ni, and Zn in fuel ethanol by FAAS after enrichment in column packed with 2-aminothiazole-modified silica gel. Anal Bioanal Chem. 2003;375:574–577. - PubMed
-
- Gurnani V, Singh AK, Venkataramani B. Cellulose based macromolecular chelator having pyrocatechol as an anchored ligand: synthesis and applications as metal extractant prior to their determination by flame atomic absorption spectrometry. Talanta. 2003;61(15):889–903. - PubMed
-
- Gurnani V, Singh AK, Venkataramani B. Cellulose functionalized with 8-hydroxyquinoline: new method of synthesis and applications as a solid phase extractant in the determination of metal. Anal Chim Acta. 2003;485:221–232. doi: 10.1016/S0003-2670(03)00416-1. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

