Influence of oxygen on NADH recycling and oxidative stress resistance systems in Lactobacillus panis PM1
- PMID: 23369580
- PMCID: PMC3605185
- DOI: 10.1186/2191-0855-3-10
Influence of oxygen on NADH recycling and oxidative stress resistance systems in Lactobacillus panis PM1
Abstract
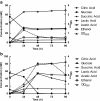
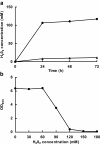
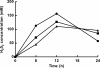
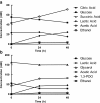
Lactobacillus panis strain PM1 is an obligatory heterofermentative and aerotolerant microorganism that also produces 1,3-propanediol from glycerol. This study investigated the metabolic responses of L. panis PM1 to oxidative stress under aerobic conditions. Growth under aerobic culture triggered an early entrance of L. panis PM1 into the stationary phase along with marked changes in end-product profiles. A ten-fold higher concentration of hydrogen peroxide was accumulated during aerobic culture compared to microaerobic culture. This H2O2 level was sufficient for the complete inhibition of L. panis PM1 cell growth, along with a significant reduction in end-products typically found during anaerobic growth. In silico analysis revealed that L. panis possessed two genes for NADH oxidase and NADH peroxidase, but their expression levels were not significantly affected by the presence of oxygen. Specific activities for these two enzymes were observed in crude extracts from L. panis PM1. Enzyme assays demonstrated that the majority of the H2O2 in the culture media was the product of NADH: H2O2 oxidase which was constitutively-active under both aerobic and microaerobic conditions; whereas, NADH peroxidase was positively-activated by the presence of oxygen and had a long induction time in contrast to NADH oxidase. These observations indicated that a coupled NADH oxidase - NADH peroxidase system was the main oxidative stress resistance mechanism in L. panis PM1, and was regulated by oxygen availability. Under aerobic conditions, NADH is mainly reoxidized by the NADH oxidase - peroxidase system rather than through the production of ethanol (or 1,3-propanediol or succinic acid production if glycerol or citric acid is available). This system helped L. panis PM1 directly use oxygen in its energy metabolism by producing extra ATP in contrast to homofermentative lactobacilli.
Figures
Similar articles
-
Metabolic engineering of a glycerol-oxidative pathway in Lactobacillus panis PM1 for utilization of bioethanol thin stillage: potential to produce platform chemicals from glycerol.Appl Environ Microbiol. 2014 Dec;80(24):7631-9. doi: 10.1128/AEM.01454-14. Epub 2014 Oct 3. Appl Environ Microbiol. 2014. PMID: 25281374 Free PMC article.
-
Contributions of citrate in redox potential maintenance and ATP production: metabolic pathways and their regulation in Lactobacillus panis PM1.Appl Microbiol Biotechnol. 2013 Oct;97(19):8693-703. doi: 10.1007/s00253-013-5108-2. Epub 2013 Aug 4. Appl Microbiol Biotechnol. 2013. PMID: 23912115
-
Isolation and characterization of novel 1,3-propanediol-producing Lactobacillus panis PM1 from bioethanol thin stillage.Appl Microbiol Biotechnol. 2013 Jan;97(1):417-28. doi: 10.1007/s00253-012-4386-4. Epub 2012 Oct 18. Appl Microbiol Biotechnol. 2013. PMID: 23076589
-
Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis.Int J Parasitol. 1998 Jan;28(1):149-64. doi: 10.1016/s0020-7519(97)00172-0. Int J Parasitol. 1998. PMID: 9504342 Review.
-
Silage review: Recent advances and future uses of silage additives.J Dairy Sci. 2018 May;101(5):3980-4000. doi: 10.3168/jds.2017-13839. J Dairy Sci. 2018. PMID: 29685273 Review.
Cited by
-
Pathogen control at the intestinal mucosa - H2O2 to the rescue.Gut Microbes. 2017 Jan 2;8(1):67-74. doi: 10.1080/19490976.2017.1279378. Epub 2017 Jan 12. Gut Microbes. 2017. PMID: 28080210 Free PMC article. Review.
-
Regulation of dual glycolytic pathways for fructose metabolism in heterofermentative Lactobacillus panis PM1.Appl Environ Microbiol. 2013 Dec;79(24):7818-26. doi: 10.1128/AEM.02377-13. Epub 2013 Oct 4. Appl Environ Microbiol. 2013. PMID: 24096428 Free PMC article.
-
Metabolic Profiling and Cold-Starvation Stress Response of Oxygen-Tolerant Lactobacillus gasseri Strains Cultured in Batch Bioreactor.Microorganisms. 2019 Jul 15;7(7):200. doi: 10.3390/microorganisms7070200. Microorganisms. 2019. PMID: 31311070 Free PMC article.
-
Metabolic engineering of a glycerol-oxidative pathway in Lactobacillus panis PM1 for utilization of bioethanol thin stillage: potential to produce platform chemicals from glycerol.Appl Environ Microbiol. 2014 Dec;80(24):7631-9. doi: 10.1128/AEM.01454-14. Epub 2014 Oct 3. Appl Environ Microbiol. 2014. PMID: 25281374 Free PMC article.
-
Assessment of aerobic and respiratory growth in the Lactobacillus casei group.PLoS One. 2014 Jun 11;9(6):e99189. doi: 10.1371/journal.pone.0099189. eCollection 2014. PLoS One. 2014. PMID: 24918811 Free PMC article.
References
-
- Chen K-H, McFeeters RF. Utilization of electron acceptors for anaerobic metabolism by Lactobacillus plantarum. Enzymes and intermediates in the utilization of citrate. Food microbiology. 1986;3:83–92.
-
- Condon S. Responses of lactic acid bacteria to oxyben. FEMS Microbiol Rev. 1987;46:269–280. doi: 10.1111/j.1574-6968.1987.tb02465.x. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

