Alternative approaches to myeloid suppressor cell therapy in transplantation: comparing regulatory macrophages to tolerogenic DCs and MDSCs
- PMID: 23369628
- PMCID: PMC3561050
- DOI: 10.1186/2047-1440-1-17
Alternative approaches to myeloid suppressor cell therapy in transplantation: comparing regulatory macrophages to tolerogenic DCs and MDSCs
Abstract
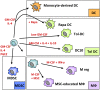
Several types of myeloid suppressor cell are currently being developed as cell-based immunosuppressive agents. Despite detailed knowledge about the molecular and cellular functions of these cell types, expert opinions differ on how to best implement such therapies in solid organ transplantation. Efforts in our laboratory to develop a cell-based medicinal product for promoting tolerance in renal transplant patients have focused on a type of suppressor macrophage, which we call the regulatory macrophage (M reg). Our favoured clinical strategy is to administer donor-derived M regs to recipients one week prior to transplantation. In contrast, many groups working with tolerogenic dendritic cells (DCs) advocate post-transplant administration of recipient-derived cells. A third alternative, using myeloid-derived suppressor cells, presumably demands that cells are given around the time of transplantation, so that they can infiltrate the graft to create a suppressive environment. On present evidence, it is not possible to say which cell type and treatment strategy might be clinically superior. This review seeks to position our basic scientific and early-stage clinical studies of human regulatory macrophages within the broader context of myeloid suppressor cell therapy in transplantation.
Figures



References
-
- Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, Oberg HH, Pascher A, Lutzen U, Janssen U. et al.Cutting Edge: immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011;187:2072–2078. doi: 10.4049/jimmunol.1100762. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

