A Rab10-dependent mechanism for polarized basement membrane secretion during organ morphogenesis
- PMID: 23369713
- PMCID: PMC3562474
- DOI: 10.1016/j.devcel.2012.12.005
A Rab10-dependent mechanism for polarized basement membrane secretion during organ morphogenesis
Abstract
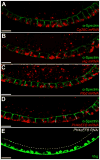
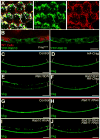
Basement membranes (BMs) are specialized extracellular matrices that are essential for epithelial structure and morphogenesis. However, little is known about how BM proteins are delivered to the basal cell surface or how this process is regulated during development. Here, we identify a mechanism for polarized BM secretion in the Drosophila follicle cells. BM proteins are synthesized in a basal endoplasmic reticulum (ER) compartment from localized mRNAs and are then exported through Tango1-positive ER exit sites to basal Golgi clusters. Next, Crag targets Rab10 to structures in the basal cytoplasm, where it restricts protein delivery to the basal surface. These events occur during egg chamber elongation, a morphogenetic process that depends on follicle cell planar polarity and BM remodeling. Significantly, Tango1 and Rab10 are also planar polarized at the basal epithelial surface. We propose that the spatial control of BM production along two tissue axes promotes exocytic efficiency, BM remodeling, and organ morphogenesis.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





References
-
- Arnaoutova I, George J, Kleinman HK, Benton G. Basement membrane matrix (BME) has multiple uses with stem cells. Stem Cell Rev. 2012;8:163–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

