Anticancer efficacy of cisplatin and trichostatin A or 5-aza-2'-deoxycytidine on ovarian cancer
- PMID: 23370212
- PMCID: PMC3593556
- DOI: 10.1038/bjc.2013.10
Anticancer efficacy of cisplatin and trichostatin A or 5-aza-2'-deoxycytidine on ovarian cancer
Abstract
Background: To evaluate the anticancer efficacy of the combination of epigenetic modifiers and cisplatin in human ovarian cancer.
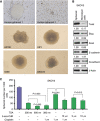
Methods: The effect of trichostatin A (TSA) and 5-aza-2'-deoxycytidine alone or in combination with low-dose cisplatin was evaluated on human ovarian cancer cell lines in vitro. We measured drug interaction by MTS assay, migration by transwell assay, expression of epithelial to mesenchymal transition (EMT) markers (Twist, Snail, Slug, E-cadherin, and N-cadherin), pluripotency markers (Oct4, Sox2, and Nanog), and epigenetic markers (DNMT3A, LSD1 and H3K4me2, H3K4me3, H3K9me2, and H3K9me3) by western blot, and the impact on and characteristics of spheroid growth when exposed to these drugs. Mouse xenografts were used to evaluate the anticancer effect of sequential drug treatment.
Results: Combination treatment had greater efficacy than single drugs and significantly suppressed cell viability, migration, and spheroid formation and growth. Sequential treatment of cisplatin (1 mg kg(-1)) followed by TSA (0.3 mg kg(-1)) significantly suppressed tumorigenicity of HEY xenografts through inhibition of EMT and decreased pluripotency of ovarian cancer cells.
Conclusion: Epigenetic modifiers potentiate the anticancer efficacy of low-dose cisplatin in ovarian cancer through regulation of EMT and pluripotency, and may provide a promising treatment for ovarian cancer patients.
Figures




References
-
- Ahmed N, Thompson EW, Quinn MA. Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: an exception to the norm. J Cell Physiol. 2007;213:581–588. - PubMed
-
- Asadollahi R, Hyde CA, Zhong XY. Epigenetics of ovarian cancer: from the lab to the clinic. Gynecol Oncol. 2010;118:81–87. - PubMed
-
- Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, Marks JR, Berchuck A, Murphy SK. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–218. - PubMed
-
- Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–3029. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

