High proportions of regulatory B and T cells are associated with decreased cellular responses to pH1N1 influenza vaccine in HIV-infected children and youth (IMPAACT P1088)
- PMID: 23370281
- PMCID: PMC3899162
- DOI: 10.4161/hv.23774
High proportions of regulatory B and T cells are associated with decreased cellular responses to pH1N1 influenza vaccine in HIV-infected children and youth (IMPAACT P1088)
Abstract
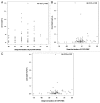
HIV-infected individuals have poor responses to inactivated influenza vaccines. To evaluate the potential role of regulatory T (Treg) and B cells (Breg), we analyzed their correlation with humoral and cell-mediated immune (CMI) responses to pandemic influenza (pH1N1) monovalent vaccine in HIV-infected children and youth. Seventy-four HIV-infected, 4- to 25-y old participants in a 2-dose pH1N1 vaccine study had circulating and pH1N1-stimulated Treg and Breg measured by flow cytometry at baseline, post-dose 1 and post-dose 2. Concomitantly, CMI was measured by ELISPOT and flow cytometry; and antibodies by hemagglutination inhibition (HAI). At baseline, most of the participants had pH1N1-specific IFNγ ELISPOT responses, whose magnitude positively correlated with the baseline pH1N1, but not with seasonal H1N1 HAI titers. pH1N1-specific IFNγ ELISPOT responses did not change post-dose 1 and significantly decreased post-dose 2. In contrast, circulating CD4+CD25+% and CD4+FOXP3+% Treg increased after vaccination. The decrease in IFNγ ELISPOT results was marginally associated with higher pH1N1-specific CD19+FOXP3+ and CD4+TGFβ+% Breg and Treg, respectively. In contrast, increases in HAI titers post-dose 1 were associated with significantly higher circulating CD19+CD25+% post-dose 1, whereas increases in IFNγ ELISPOT results post-dose 1 were associated with higher circulating CD4+/C8+CD25+FOXP3+%. In conclusion, in HIV-infected children and youth, influenza-specific Treg and Breg may contribute to poor responses to vaccination. However, robust humoral and CMI responses to vaccination may result in increased circulating Treg and/or Breg, establishing a feed-back mechanism.
Keywords: HIV infection; cell-mediated immunity; influenza vaccine; regulatory B cells; regulatory T cells.
Figures
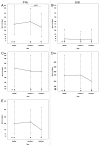

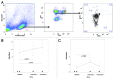
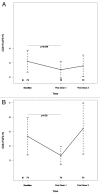
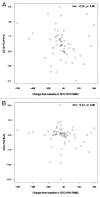
References
-
- Levin MJ, Song LY, Fenton T, Nachman S, Patterson J, Walker R, et al. Shedding of live vaccine virus, comparative safety, and influenza-specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV-infected children. Vaccine. 2008;26:4210–7. doi: 10.1016/j.vaccine.2008.05.054. - DOI - PMC - PubMed
-
- Cooper C, Thorne A, Klein M, Conway B, Boivin G, Haase D, et al. CIHR Canadian HIV Trials Network Influenza Vaccine Research Group Immunogenicity is not improved by increased antigen dose or booster dosing of seasonal influenza vaccine in a randomized trial of HIV infected adults. PLoS One. 2011;6:e17758. doi: 10.1371/journal.pone.0017758. - DOI - PMC - PubMed
-
- Crum-Cianflone NF, Eberly LE, Duplessis C, Maguire J, Ganesan A, Faix D, et al. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in an immunocompromised population: a prospective study comparing HIV-infected adults with HIV-uninfected adults. Clin Infect Dis. 2011;52:138–46. doi: 10.1093/cid/ciq019. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR029890/RR/NCRR NIH HHS/United States
- HHSN267200800001G/DK/NIDDK NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- UM1 AI069415/AI/NIAID NIH HHS/United States
- UM1 AI069477/AI/NIAID NIH HHS/United States
- U01 AI068616/AI/NIAID NIH HHS/United States
- HHSN267200800001C/HD/NICHD NIH HHS/United States
- U01 AI041110/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- UL1 RR031982/RR/NCRR NIH HHS/United States
- UM1 AI069536/AI/NIAID NIH HHS/United States
- UL1 TR000154/TR/NCATS NIH HHS/United States
- UL1 RR029893/RR/NCRR NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
