The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs
- PMID: 23370617
- PMCID: PMC4115188
- DOI: 10.1136/jnnp-2012-304270
The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs
Abstract
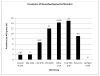
The aim of this study was to compare the prevalence of diagnosed neurodevelopmental disorders in children exposed, in utero, to different antiepileptic drug treatments. A prospective cohort of women with epilepsy and a control group of women without epilepsy were recruited from antenatal clinics. The children of this cohort were followed longitudinally until 6 years of age (n=415). Diagnosis of a neurodevelopmental disorder was made independently of the research team. Multiple logistic regression analysis revealed an increase in risk of neurodevelopmental disorders in children exposed to monotherapy sodium valproate (VPA) (6/50, 12.0%; aOR 6.05, 95%CI 1.65 to 24.53, p=0.007) and in those exposed to polytherapy with sodium VPA (3/20, 15.0%; aOR 9.97, 95% CI 1.82 to 49.40, p=0.005) compared with control children (4/214; 1.87%). Autistic spectrum disorder was the most frequent diagnosis. No significant increase was found among children exposed to carbamazepine (1/50) or lamotrigine (2/30). An accumulation of evidence demonstrates that the risks associated with prenatal sodium VPA exposure include an increased prevalence of neurodevelopmental disorders. Whether such disorders are discrete or represent the severe end of a continuum of altered neurodevelopmental functioning requires further investigation. Replication and extension of this research is required to investigate the mechanism(s) underpinning the relationship. Finally, the increased likelihood of neurodevelopmental disorders should be communicated to women for whom sodium VPA is a treatment option.
Keywords: Epilepsy; Neuropsychology; Neurotoxicology; Obstetrics; Paediatric.
Conflict of interest statement
Dr Bromley has received honorarium from Sanofi-Aventis for presenting to their Advisory Panel on two occasions. Dr Bromley has also provided expert testimony regarding fetal exposure to antiepileptic drugs. Travel support for two conferences has also been received from UCB Pharma and she has worked on projects supported by Educational grants from Sanofi Aventis and UCB Pharma.
Professor Mawer has provided expert testimony regarding fetal exposure to antiepileptic drugs.
Ms Briggs has no disclosures.
Dr Cheyne has no disclosures.
Professor Jill Clayton-Smith has provided expert testimony regarding fetal exposure to antiepileptic drugs and has received honorarium from GSK.
Dr García-Fiñana has no disclosures.
Dr Kneen has no disclosures.
Dr Lucas has no disclosures.
Dr Shallcross has received honorarium from UCB pharma for a lecture and also travel support from UCB pharma for conference attendance.
Professor Baker has received educational grants for this research from Sanofi-Aventis and has also received an educational grant from UCB Pharma for work not reported here. Professor Baker has also provided expert testimony regarding fetal exposure to antiepileptic drugs and has received honorarium from UCB pharma, GSK and Sanofi Aventis for lectures given.
Figures
References
-
- Tomson T, Battino D, Bonizzoni E, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurology. 2011;10:609–617. - PubMed
-
- Bromley RL, Mawer G, Love J, et al. Early cognitive development in children born to women with epilepsy: A prospective report. Epilepsia. 2010 Jul 14; - PubMed
-
- Stromland K, Nordin V, Miller M, Akerstrom B, Gillberg C. Autism in Thalidomide Embryopathy: A Population Study. Developmental Medicine and Child Neurology. 1994;36:351–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical