Electrophysiological characteristics of enteric neurons isolated from the immortomouse
- PMID: 23371009
- PMCID: PMC3664262
- DOI: 10.1007/s10620-013-2557-5
Electrophysiological characteristics of enteric neurons isolated from the immortomouse
Abstract
Background: Recently, two enteric neuronal cell lines, one fetal and the other post-natal (IM-PEN), have been developed from the H-2K(b)-tsA58 transgenic mouse (immortomouse). However, their electrophysiological properties are not known. The goal of this study was to determine the electrical excitability and ionic conductance of the immortalized postnatal enteric neuronal (IM-PEN) cell line.
Methods: Whole cell patch clamp studies, immunohistochemistry and RT-PCR were performed on differentiated IM-PEN cells following propagation at 33 °C and differentiation at 37 °C.
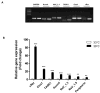
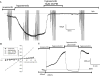
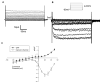
Results: Differentiated IM-PEN cells stained positively for the neuron specific markers βIII-tubulin and PGP9.5. The mRNA for several ion channels expressed in enteric neurons were detected by RT-PCR. In current clamp, the resting membrane potential was -24.6 ± 2.1 mV (n = 6) for IM-FEN and -29.8 ± 0.9 mV (n = 30) for IM-PEN. Current injections from Vh -80 mV resulted in passive responses but not action potentials. Depolarizing pulses in the whole cell voltage clamp configuration from Vh -80 mV elicited small nifedipine-sensitive inward currents. Additionally, outward currents with slow deactivating tail currents were blocked by niflumic acid and low chloride solution. A volume-regulated anion current was elicited by hypo-osmotic solution and inhibited by 10 μM DCPIB. Growth with rabbit gastrointestinal smooth muscle did not yield significant differences in the active properties of the IM-PEN cell line. Transient expression of L-type Ca(2+) channels produced large inward currents demonstrating a working mechanism for protein folding and transport.
Conclusion: The electrophysiological characteristics of IM-PEN cells suggest that chloride channels in IM-PEN cells play an important role in their resting state, and membrane trafficking of some of the ion channels may preclude their electrical excitability.
Conflict of interest statement
Figures






Similar articles
-
Molecular and functional analysis of hyperpolarisation-activated nucleotide-gated (HCN) channels in the enteric nervous system.Neuroscience. 2004;129(3):603-14. doi: 10.1016/j.neuroscience.2004.08.027. Neuroscience. 2004. PMID: 15541882
-
Ionic channel mechanisms mediating the intrinsic excitability of Kenyon cells in the mushroom body of the cricket brain.J Insect Physiol. 2014 Sep;68:44-57. doi: 10.1016/j.jinsphys.2014.06.013. Epub 2014 Jul 1. J Insect Physiol. 2014. PMID: 24995840
-
Characteristics of action potentials and their underlying outward currents in rat taste receptor cells.J Neurophysiol. 1996 Feb;75(2):820-31. doi: 10.1152/jn.1996.75.2.820. J Neurophysiol. 1996. PMID: 8714655
-
Passive and active membrane properties of isolated rat intracardiac neurons: regulation by H- and M-currents.J Neurophysiol. 1997 Oct;78(4):1890-902. doi: 10.1152/jn.1997.78.4.1890. J Neurophysiol. 1997. PMID: 9325358
-
Afterhyperpolarization current in myenteric neurons of the guinea pig duodenum.J Neurophysiol. 2001 May;85(5):1941-51. doi: 10.1152/jn.2001.85.5.1941. J Neurophysiol. 2001. PMID: 11353011
Cited by
-
A functional network of highly pure enteric neurons in a dish.Front Neurosci. 2023 Jan 6;16:1062253. doi: 10.3389/fnins.2022.1062253. eCollection 2022. Front Neurosci. 2023. PMID: 36685225 Free PMC article.
-
High Capability of Pentagalloylglucose (PGG) in Inhibiting Multiple Types of Membrane Ionic Currents.Int J Mol Sci. 2020 Dec 9;21(24):9369. doi: 10.3390/ijms21249369. Int J Mol Sci. 2020. PMID: 33316951 Free PMC article.
-
Volume-regulated anion channel--a frenemy within the brain.Pflugers Arch. 2016 Mar;468(3):421-41. doi: 10.1007/s00424-015-1765-6. Epub 2015 Dec 1. Pflugers Arch. 2016. PMID: 26620797 Free PMC article. Review.
-
Best practice for passaging murine embryonic enteric neuronal cell line before differentiation.Cytotechnology. 2016 Dec;68(6):2379-2388. doi: 10.1007/s10616-016-9953-6. Epub 2016 Feb 24. Cytotechnology. 2016. PMID: 26910417 Free PMC article.
References
-
- Mongardi Fantaguzzi C, Thacker M, Chiocchetti R, Furness JB. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009;336:179–8. - PubMed
-
- Nurgali K, Stebbing MJ, Furness JB. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J Comp Neurol. 2004;468:112–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

