Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of "bath salts," produce opposite effects at the human dopamine transporter
- PMID: 23371489
- PMCID: PMC3881434
- DOI: 10.1007/s00213-013-2967-2
Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of "bath salts," produce opposite effects at the human dopamine transporter
Erratum in
- Psychopharmacology (Berl). 2013 Jun;227(3):447. Verkariya, Rakesh [corrected to Vekariya, Rakesh]
Abstract
Rationale: Psychoactive "bath salts" represent a relatively new drug of abuse combination that was placed in Schedule I in October 2011. Two common ingredients of bath salts include the cathinone analogs: mephedrone and methylenedioxypyrovalerone (MDPV). The mechanism of action of these synthetic cathinone analogs has not been well investigated.
Materials and methods: Because cathinone and methcathinone are known to act as releasing agents at the human dopamine transporter (hDAT), mephedrone and MDPV were investigated at hDAT expressed in Xenopus oocytes.
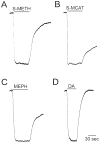
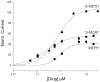
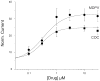
Results: Whereas mephedrone was found to have the signature of a dopamine-releasing agent similar to methamphetamine or methcathinone, MDPV behaved as a cocaine-like reuptake inhibitor of dopamine.
Conclusions: Mephedrone and MDPV produce opposite electrophysiological signatures through hDAT expressed in oocytes. Implications are that the combination (as found in bath salts) might produce effects similar to a combination of methamphetamine and cocaine.
Figures
Similar articles
-
Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter.Br J Pharmacol. 2013 Apr;168(7):1750-7. doi: 10.1111/bph.12061. Br J Pharmacol. 2013. PMID: 23170765 Free PMC article.
-
3,4-Methylenedioxypyrovalerone prevents while methylone enhances methamphetamine-induced damage to dopamine nerve endings: β-ketoamphetamine modulation of neurotoxicity by the dopamine transporter.J Neurochem. 2015 Apr;133(2):211-22. doi: 10.1111/jnc.13048. Epub 2015 Mar 2. J Neurochem. 2015. PMID: 25626880 Free PMC article.
-
Pharmacology of novel synthetic stimulants structurally related to the "bath salts" constituent 3,4-methylenedioxypyrovalerone (MDPV).Neuropharmacology. 2014 Dec;87:206-13. doi: 10.1016/j.neuropharm.2014.02.016. Epub 2014 Mar 2. Neuropharmacology. 2014. PMID: 24594476 Free PMC article.
-
Neurotoxicology of Synthetic Cathinone Analogs.Curr Top Behav Neurosci. 2017;32:209-230. doi: 10.1007/7854_2016_21. Curr Top Behav Neurosci. 2017. PMID: 27753008 Free PMC article. Review.
-
DARK Classics in Chemical Neuroscience: Cathinone-Derived Psychostimulants.ACS Chem Neurosci. 2018 Oct 17;9(10):2379-2394. doi: 10.1021/acschemneuro.8b00147. Epub 2018 May 11. ACS Chem Neurosci. 2018. PMID: 29714473 Free PMC article. Review.
Cited by
-
Reinforcing Effects of Cathinone NPS in the Intravenous Drug Self-Administration Paradigm.Curr Top Behav Neurosci. 2017;32:133-143. doi: 10.1007/7854_2016_33. Curr Top Behav Neurosci. 2017. PMID: 27431398 Review.
-
Neuropharmacological and Neurogenetic Correlates of Opioid Use Disorder (OUD) As a Function of Ethnicity: Relevance to Precision Addiction Medicine.Curr Neuropharmacol. 2020;18(7):578-595. doi: 10.2174/1570159X17666191118125702. Curr Neuropharmacol. 2020. PMID: 31744450 Free PMC article. Review.
-
Synthetic Cathinones: A Brief Overview of Overviews with Applications to the Forensic Sciences.Ann Forensic Res Anal. 2017;4(2):1040. Epub 2017 Mar 21. Ann Forensic Res Anal. 2017. PMID: 30288398 Free PMC article.
-
Structure-activity relationships of bath salt components: substituted cathinones and benzofurans at biogenic amine transporters.Psychopharmacology (Berl). 2019 Mar;236(3):939-952. doi: 10.1007/s00213-018-5059-5. Epub 2018 Nov 5. Psychopharmacology (Berl). 2019. PMID: 30397775 Free PMC article.
-
Alpha-pyrrolidinopentiophenone and mephedrone self-administration produce differential neurochemical changes following short- or long-access conditions in rats.Eur J Pharmacol. 2021 Apr 15;897:173935. doi: 10.1016/j.ejphar.2021.173935. Epub 2021 Feb 10. Eur J Pharmacol. 2021. PMID: 33577836 Free PMC article.
References
-
- Balint EE, Falkay G, Balint GA. Khat – A controversial plant. Wein Klin Wochenschr. 2009;121:604–614. - PubMed
-
- Dal Cason TA, Young R, Glennon RA. Cathinone: An investigation of several N-alkyl and methylenedioxy analogs. Pharmacol Biochem Behav. 1997;58:1109–1116. - PubMed
-
- de Durnaga S, Sanchez J. A homolog of ephedrine. Bull Soc Chim Fr. 1929;45:284–286.
-
- DeFelice LJ, Goswami T. Transporters as channels. Annu Rev Physiol. 2007;69:87–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

