Immunogenicity of pluripotent stem cells and their derivatives
- PMID: 23371903
- PMCID: PMC3638957
- DOI: 10.1161/CIRCRESAHA.111.249243
Immunogenicity of pluripotent stem cells and their derivatives
Abstract
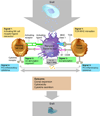
The ability of pluripotent stem cells to self-renew and differentiate into all somatic cell types brings great prospects to regenerative medicine and human health. However, before clinical applications, much translational research is necessary to ensure that their therapeutic progenies are functional and nontumorigenic, that they are stable and do not dedifferentiate, and that they do not elicit immune responses that could threaten their survival in vivo. For this, an in-depth understanding of their biology, genetic, and epigenetic make-up and of their antigenic repertoire is critical for predicting their immunogenicity and for developing strategies needed to assure successful long-term engraftment. Recently, the expectation that reprogrammed somatic cells would provide an autologous cell therapy for personalized medicine has been questioned. Induced pluripotent stem cells display several genetic and epigenetic abnormalities that could promote tumorigenicity and immunogenicity in vivo. Understanding the persistence and effects of these abnormalities in induced pluripotent stem cell derivatives is critical to allow clinicians to predict graft fate after transplantation, and to take requisite measures to prevent immune rejection. With clinical trials of pluripotent stem cell therapy on the horizon, the importance of understanding immunologic barriers and devising safe, effective strategies to bypass them is further underscored. This approach to overcome immunologic barriers to stem cell therapy can take advantage of the validated knowledge acquired from decades of hematopoietic stem cell transplantation.
Figures




References
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
-
- Reubinoff BE, Pera MF, Fong C-Y, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotech. 2000;18:399–404. - PubMed
-
- Soundararajan P, Lindsey BW, Leopold C, Rafuse VF. Easy and rapid differentiation of embryonic stem cells into functional motoneurons using sonic hedgehog-producing cells. Stem Cells. 2007;25:1697–1706. - PubMed
-
- Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1:495–507. - PubMed
-
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

