Progress in the reprogramming of somatic cells
- PMID: 23371904
- PMCID: PMC3790469
- DOI: 10.1161/CIRCRESAHA.111.249235
Progress in the reprogramming of somatic cells
Abstract
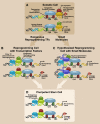
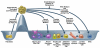
Pluripotent stem cells can differentiate into nearly all types of cells in the body. This unique potential provides significant promise for cell-based therapies to restore tissues or organs destroyed by injuries, degenerative diseases, aging, or cancer. The discovery of induced pluripotent stem cell (iPSC) technology offers a possible strategy to generate patient-specific pluripotent stem cells. However, because of concerns about the specificity, efficiency, kinetics, and safety of iPSC reprogramming, improvements or fundamental changes in this process are required before their effective clinical use. A chemical approach is regarded as a promising strategy to improve and change the iPSC process. Dozens of small molecules have been identified that can functionally replace reprogramming factors and significantly improve iPSC reprogramming. In addition to the prospect of deriving patient-specific tissues and organs from iPSCs, another attractive strategy for regenerative medicine is transdifferentiation-the direct conversion of one somatic cell type to another. Recent studies revealed a new paradigm of transdifferentiation: using transcription factors used in iPSC generation to induce transdifferentiation or called iPSC transcription factor-based transdifferentiation. This type of transdifferentiation not only reveals and uses the developmentally plastic intermediates generated during iPSC reprogramming but also produces a wide range of cells, including expandable tissue-specific precursor cells. Here, we review recent progress of small molecule approaches in the generation of iPSCs. In addition, we summarize the new concept of iPSC transcription factor-based transdifferentiation and discuss its application in generating various lineage-specific cells, especially cardiovascular cells.
Figures
Similar articles
-
Pharmacological Reprogramming of Somatic Cells for Regenerative Medicine.Acc Chem Res. 2017 May 16;50(5):1202-1211. doi: 10.1021/acs.accounts.7b00020. Epub 2017 Apr 28. Acc Chem Res. 2017. PMID: 28453285
-
Chemically Induced Reprogramming of Somatic Cells to Pluripotent Stem Cells and Neural Cells.Int J Mol Sci. 2016 Feb 6;17(2):226. doi: 10.3390/ijms17020226. Int J Mol Sci. 2016. PMID: 26861316 Free PMC article. Review.
-
Effect of small molecules on cell reprogramming.Mol Biosyst. 2017 Jan 31;13(2):277-313. doi: 10.1039/c6mb00595k. Mol Biosyst. 2017. PMID: 27918060 Review.
-
Small molecules for reprogramming and transdifferentiation.Cell Mol Life Sci. 2017 Oct;74(19):3553-3575. doi: 10.1007/s00018-017-2586-x. Epub 2017 Jul 11. Cell Mol Life Sci. 2017. PMID: 28698932 Free PMC article. Review.
-
RNA-based tools for nuclear reprogramming and lineage-conversion: towards clinical applications.J Cardiovasc Transl Res. 2013 Dec;6(6):956-68. doi: 10.1007/s12265-013-9494-8. Epub 2013 Jul 13. J Cardiovasc Transl Res. 2013. PMID: 23852582 Free PMC article. Review.
Cited by
-
Direct conversion of human fibroblasts into therapeutically active vascular wall-typical mesenchymal stem cells.Cell Mol Life Sci. 2020 Sep;77(17):3401-3422. doi: 10.1007/s00018-019-03358-0. Epub 2019 Nov 11. Cell Mol Life Sci. 2020. PMID: 31712992 Free PMC article.
-
Human iPSC-Based Models for the Development of Therapeutics Targeting Neurodegenerative Lysosomal Storage Diseases.Front Mol Biosci. 2020 Sep 18;7:224. doi: 10.3389/fmolb.2020.00224. eCollection 2020. Front Mol Biosci. 2020. PMID: 33062642 Free PMC article. Review.
-
Chemically Defined Media Can Maintain Pig Pluripotency Network In Vitro.Stem Cell Reports. 2019 Jul 9;13(1):221-234. doi: 10.1016/j.stemcr.2019.05.028. Epub 2019 Jun 27. Stem Cell Reports. 2019. PMID: 31257130 Free PMC article.
-
Cellular reprogramming for clinical cartilage repair.Cell Biol Toxicol. 2017 Aug;33(4):329-349. doi: 10.1007/s10565-017-9382-0. Epub 2017 Jan 31. Cell Biol Toxicol. 2017. PMID: 28144824 Free PMC article. Review.
-
Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening.Biotechnol Adv. 2017 Jan-Feb;35(1):77-94. doi: 10.1016/j.biotechadv.2016.12.002. Epub 2016 Dec 20. Biotechnol Adv. 2017. PMID: 28007615 Free PMC article. Review.
References
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

