Hepatocyte growth factor inhibits TGF-β1-induced myofibroblast differentiation in tendon fibroblasts: role of AMPK signaling pathway
- PMID: 23371911
- PMCID: PMC10718008
- DOI: 10.1007/s12576-013-0251-1
Hepatocyte growth factor inhibits TGF-β1-induced myofibroblast differentiation in tendon fibroblasts: role of AMPK signaling pathway
Erratum in
-
Correction to: Hepatocyte growth factor inhibits TGF-β1-induced myofibroblast differentiation in tendon fibroblasts: role of AMPK signaling pathway.J Physiol Sci. 2020 Oct 26;70(1):51. doi: 10.1186/s12576-020-00778-7. J Physiol Sci. 2020. PMID: 33106156 Free PMC article.
Abstract
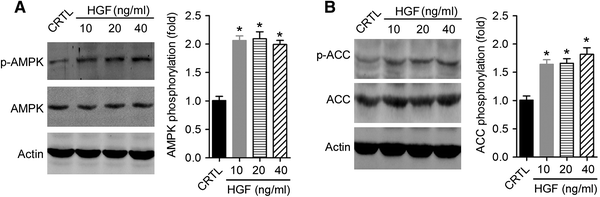
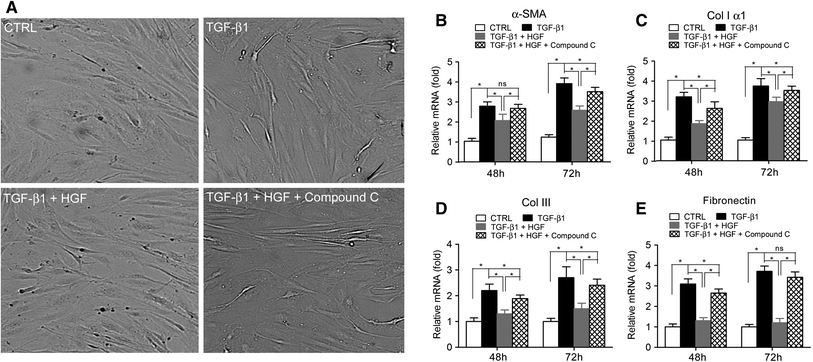
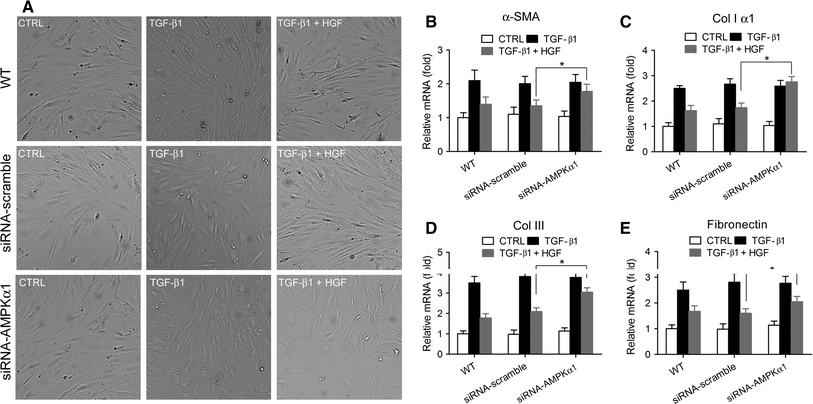
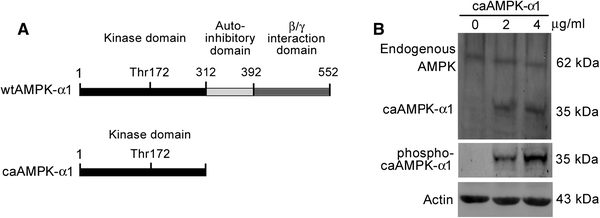
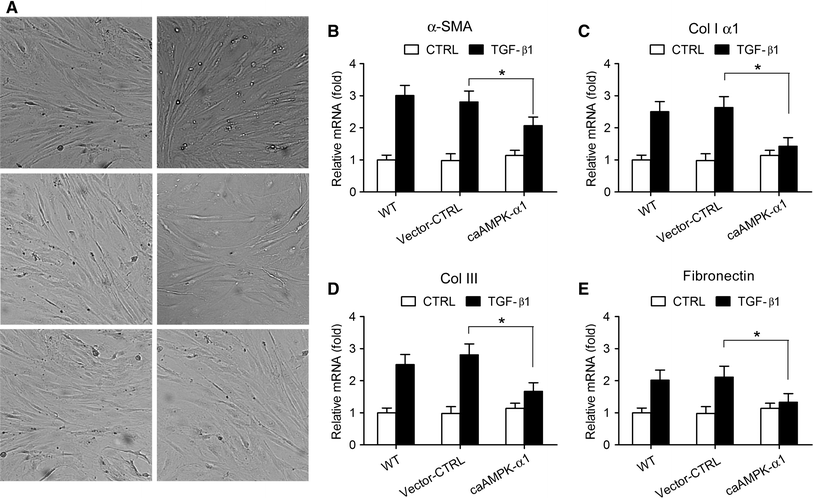
The transforming growth factor-β1 (TGF-β1)-induced myofibroblastic differentiation in tendon fibroblasts was thought to be one of the most important features of scar fibrosis formation, which is associated with occurrence of re-rupture. Previously, we reported that hepatocyte growth factor (HGF) inhibited TGF-β1-induced myofibroblast differentiation and extracellular matrix deposition in the Achilles tendon of rats. Here, we investigated the potential molecular mechanisms underlying the inhibitory effect of HGF on TGF-β1-induced myofibroblast differentiation. We found that treatment with HGF (10, 20, and 40 ng/ml) increased phosphorylation of adenosine monophosphate kinase (AMPK) and acetyl-CoA carboxylase (ACC) in tendon fibroblasts. Pharmacological inhibition of the AMPK signaling pathway using compound C, a specific blocker of AMPK signaling, remarkably attenuated the inhibitory effect of HGF on TGF-β1-induced myofibroblastic differentiation in tendon fibroblasts. Moreover, small interfering RNA (siRNA)-mediated knockdown of AMPKα1 subunit decreased the inhibitory effect of HGF on TGF-β1-induced myofibroblastic differentiation in tendon fibroblasts. Finally, overexpression of constitutively active AMPKα1, which led to constitutive activation of the AMPK signaling pathway in tendon fibroblasts, mimicked the inhibitory effect of HGF on the TGF-β1-induced myofibroblastic differentiation. Our study therefore suggests that HGF inhibits TGF-β1-induced myofibroblastic differentiation via an AMPK signaling pathway-dependent manner in tendon fibroblasts.
Conflict of interest statement
All the authors declared no conflict of interests.
Figures
Similar articles
-
HGF inhibits TGF-β1-induced myofibroblast differentiation and ECM deposition via MMP-2 in Achilles tendon in rat.Eur J Appl Physiol. 2011 Jul;111(7):1457-63. doi: 10.1007/s00421-010-1764-4. Epub 2010 Dec 17. Eur J Appl Physiol. 2011. PMID: 21165643
-
Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction.Am J Pathol. 2003 Aug;163(2):621-32. doi: 10.1016/S0002-9440(10)63689-9. Am J Pathol. 2003. PMID: 12875981 Free PMC article.
-
Inhibitory effects of hepatocyte growth factor and interleukin-6 on transforming growth factor-beta1 mediated vocal fold fibroblast-myofibroblast differentiation.Ann Otol Rhinol Laryngol. 2010 May;119(5):350-7. doi: 10.1177/000348941011900513. Ann Otol Rhinol Laryngol. 2010. PMID: 20524582 Free PMC article.
-
CTRP3 attenuates post-infarct cardiac fibrosis by targeting Smad3 activation and inhibiting myofibroblast differentiation.J Mol Med (Berl). 2015 Dec;93(12):1311-25. doi: 10.1007/s00109-015-1309-8. Epub 2015 Jul 3. J Mol Med (Berl). 2015. PMID: 26138247
-
The AMPK agonist AICAR inhibits TGF-β1 induced activation of kidney myofibroblasts.PLoS One. 2014 Sep 4;9(9):e106554. doi: 10.1371/journal.pone.0106554. eCollection 2014. PLoS One. 2014. PMID: 25188319 Free PMC article.
Cited by
-
Multifunctional applications and research advances of low-molecular-weight heparin.Front Pharmacol. 2025 May 21;16:1585762. doi: 10.3389/fphar.2025.1585762. eCollection 2025. Front Pharmacol. 2025. PMID: 40469990 Free PMC article. Review.
-
Tendon healing: a concise review on cellular and molecular mechanisms with a particular focus on the Achilles tendon.Bone Joint Res. 2022 Aug;11(8):561-574. doi: 10.1302/2046-3758.118.BJR-2021-0576.R1. Bone Joint Res. 2022. PMID: 35920195 Free PMC article.
-
Serum Glycoproteome Profiles for Distinguishing Intestinal Fibrosis from Inflammation in Crohn's Disease.PLoS One. 2017 Jan 23;12(1):e0170506. doi: 10.1371/journal.pone.0170506. eCollection 2017. PLoS One. 2017. PMID: 28114331 Free PMC article.
-
Correction to: Hepatocyte growth factor inhibits TGF-β1-induced myofibroblast differentiation in tendon fibroblasts: role of AMPK signaling pathway.J Physiol Sci. 2020 Oct 26;70(1):51. doi: 10.1186/s12576-020-00778-7. J Physiol Sci. 2020. PMID: 33106156 Free PMC article.
-
[Association between hepatocyte growth factor in tears and corneal haze in rabbits early after epipolis laser in situ keratomileusis].Nan Fang Yi Ke Da Xue Xue Bao. 2017 Nov 20;37(11):1551-1554. doi: 10.3969/j.issn.1673-4254.2017.11.21. Nan Fang Yi Ke Da Xue Xue Bao. 2017. PMID: 29180340 Free PMC article. Chinese.
References
-
- Hamilton JA, Butler DM, Stanton H. Cytokine interactions promoting DNA synthesis in human synovial fibroblasts. J Rheumatol. 1994;21:797–803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

