A developmental framework for complex plasmodesmata formation revealed by large-scale imaging of the Arabidopsis leaf epidermis
- PMID: 23371949
- PMCID: PMC3584549
- DOI: 10.1105/tpc.112.105890
A developmental framework for complex plasmodesmata formation revealed by large-scale imaging of the Arabidopsis leaf epidermis
Abstract
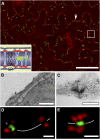
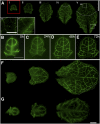
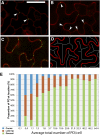
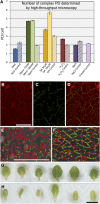
Plasmodesmata (PD) form tubular connections that function as intercellular communication channels. They are essential for transporting nutrients and for coordinating development. During cytokinesis, simple PDs are inserted into the developing cell plate, while during wall extension, more complex (branched) forms of PD are laid down. We show that complex PDs are derived from existing simple PDs in a pattern that is accelerated when leaves undergo the sink-source transition. Complex PDs are inserted initially at the three-way junctions between epidermal cells but develop most rapidly in the anisocytic complexes around stomata. For a quantitative analysis of complex PD formation, we established a high-throughput imaging platform and constructed PDQUANT, a custom algorithm that detected cell boundaries and PD numbers in different wall faces. For anticlinal walls, the number of complex PDs increased with increasing cell size, while for periclinal walls, the number of PDs decreased. Complex PD insertion was accelerated by up to threefold in response to salicylic acid treatment and challenges with mannitol. In a single 30-min run, we could derive data for up to 11k PDs from 3k epidermal cells. This facile approach opens the door to a large-scale analysis of the endogenous and exogenous factors that influence PD formation.
Figures




References
-
- Burch-Smith T.M., Zambryski P.C. (2012). Plasmodesmata paradigm shift: Regulation from without versus within. Annu. Rev. Plant Biol. 63: 239–260 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

