Novel sublingual low-dose zolpidem tablet reduces latency to sleep onset following spontaneous middle-of-the-night awakening in insomnia in a randomized, double-blind, placebo-controlled, outpatient study
- PMID: 23372266
- PMCID: PMC3543068
- DOI: 10.5665/sleep.2370
Novel sublingual low-dose zolpidem tablet reduces latency to sleep onset following spontaneous middle-of-the-night awakening in insomnia in a randomized, double-blind, placebo-controlled, outpatient study
Abstract
Study objectives: To evaluate efficacy and safety of 3.5-mg zolpidem tartrate sublingual tablets (ZST) on latency to sleep onset after middle-of-the-night (MOTN) awakenings in patients with insomnia characterized by difficulty returning to sleep after MOTN awakenings.
Design: Multicenter randomized, double-blind, placebo-controlled, parallel-group.
Setting: Outpatient.
Patients: There were 295 adults (median age 43 y; 68.1% female) with primary insomnia and difficulty returning to sleep after MOTN awakenings (three or more MOTN awakenings/wk during screening).
Interventions: After a 2-wk, single-blind placebo eligibility period, participants were randomized 1:1 to as-needed MOTN dosing with 3.5 mg ZST or placebo for 28 nights. An interactive voice response system determined if the study drug could be taken and recorded sleep/wake efficacy measures.
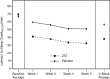
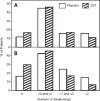
Results: ZST significantly (P < 0.0001) decreased latency to sleep onset over 4 wk (baseline 68.1 min; ZST 38.2 min) compared with placebo (baseline 69.4 min; placebo 56.4 min). Ratings of morning sleepiness/alertness significantly (P = 0.0041) favored the ZST group on nights medication was taken but not on other nights. Participants in the ZST group took the study drug on 62% of nights during the 4 wk; members of the placebo group took study medication on 64% of nights. Adverse events were generally mild and at the same rate (19.3% of participants) in both groups. There were no treatment-related serious adverse events (SAEs), and one adverse event-related study discontinuation from the placebo group. Dosing/week did not increase across the study.
Conclusions: 3.5 mg ZST used as needed significantly reduced latency to return to sleep in comparison with placebo in these patients with insomnia. Sleep quality was improved, and morning sleepiness/alertness scores also improved. ZST was well tolerated. These data demonstrate the utility of a sleep-promoting agent when used as needed in the MOTN.
Clinical trials registration: NCT00466193: "A Study of Zolpidem Tartrate Tablet in Adult Patients with Insomnia" http://www.clinicaltrials.gov/ct2/show/NCT00466193?spons=%22Transcept+Pharmaceuticals%22&spons_ex=Y&rank=2
Figures


Similar articles
-
Low-dose sublingual zolpidem tartrate is associated with dose-related improvement in sleep onset and duration in insomnia characterized by middle-of-the-night (MOTN) awakenings.Sleep. 2008 Sep;31(9):1277-84. Sleep. 2008. PMID: 18788653 Free PMC article. Clinical Trial.
-
Gender influences on efficacy and safety of sublingual zolpidem tartrate for middle-of-the-night awakening in insomnia.Hum Psychopharmacol. 2014 Jan;29(1):25-30. doi: 10.1002/hup.2364. Epub 2013 Nov 7. Hum Psychopharmacol. 2014. PMID: 24424704 Clinical Trial.
-
Comparison of Lemborexant With Placebo and Zolpidem Tartrate Extended Release for the Treatment of Older Adults With Insomnia Disorder: A Phase 3 Randomized Clinical Trial.JAMA Netw Open. 2019 Dec 2;2(12):e1918254. doi: 10.1001/jamanetworkopen.2019.18254. JAMA Netw Open. 2019. PMID: 31880796 Free PMC article. Clinical Trial.
-
Sublingual zolpidem (Edluar™; Sublinox™).CNS Drugs. 2012 Nov;26(11):1003-10. doi: 10.1007/s40263-012-0009-y. CNS Drugs. 2012. PMID: 23034583 Review.
-
Zolpidem's use for insomnia.Asian J Psychiatr. 2017 Feb;25:79-90. doi: 10.1016/j.ajp.2016.10.006. Epub 2016 Oct 12. Asian J Psychiatr. 2017. PMID: 28262178 Review.
Cited by
-
Residual effects of low-dose sublingual zolpidem on highway driving performance the morning after middle-of-the-night use.Sleep. 2014 Mar 1;37(3):489-96. doi: 10.5665/sleep.3482. Sleep. 2014. PMID: 24587571 Free PMC article. Clinical Trial.
-
Pharmacokinetics of zolpidem from sublingual zolpidem tartrate tablets in healthy elderly versus non-elderly subjects.Drugs Aging. 2014 Oct;31(10):731-6. doi: 10.1007/s40266-014-0211-3. Drugs Aging. 2014. PMID: 25246162 Clinical Trial.
-
2023 Guidelines on the Diagnosis and Treatment of Insomnia in Adults - Brazilian Sleep Association.Sleep Sci. 2023 Nov 22;16(Suppl 2):507-549. doi: 10.1055/s-0043-1776281. eCollection 2023 Oct. Sleep Sci. 2023. PMID: 38370879 Free PMC article. Review.
-
Challenges and Future Prospects for the Delivery of Biologics: Oral Mucosal, Pulmonary, and Transdermal Routes.AAPS J. 2017 May;19(3):652-668. doi: 10.1208/s12248-017-0054-z. Epub 2017 Feb 13. AAPS J. 2017. PMID: 28194704
-
The assessment and management of insomnia: an update.World Psychiatry. 2019 Oct;18(3):337-352. doi: 10.1002/wps.20674. World Psychiatry. 2019. PMID: 31496087 Free PMC article.
References
-
- Ohayon MM, Roth T. What are the contributing factors for insomnia in the general population? J Psychosom Res. 2001;51:745–55. - PubMed
-
- Estivill E. Behaviour of insomniacs and implication for their management. Sleep Med Rev. 2002;6:S3–6. - PubMed
-
- [Accessed August 18, 2012]. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

