Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men
- PMID: 23372274
- PMCID: PMC3542986
- DOI: 10.5665/sleep.2386
Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men
Abstract
Study objectives: Suvorexant (MK-4305) is an orexin receptor antagonist being developed for the treatment of insomnia. This report describes the effects of nighttime administration of suvorexant on polysomnography (PSG) sleep parameters in healthy young men.
Design: Randomized, double-blind, placebo-controlled, 4-period crossover PSG study, followed by an additional 5(th) period to assess pharmacokinetics.
Setting: Sleep laboratory.
Participants: Healthy young men between 18 and 45 years of age (22 enrolled, 19 completed).
Interventions: Periods 1-4: suvorexant (10 mg, 50 mg, or 100 mg) or placebo 1 h before nighttime PSG recording. Period 5: suvorexant 10 mg, 50 mg, or 100 mg.
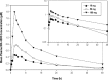
Measurements and results: In Periods 1-4, overnight sleep parameters were recorded by PSG and next-morning residual effects were assessed by psychomotor performance tests and subjective assessments. Statistically significant sleep-promoting effects were observed with all doses of suvorexant compared to placebo. Suvorexant 50 mg and 100 mg significantly decreased latency to persistent sleep and wake after sleep onset time, and increased sleep efficiency. Suvorexant 10 mg significantly decreased wake after sleep onset time. There were no statistically significant effects of suvorexant on EEG frequency bands including delta (slow wave) activity based on power spectral analysis. Suvorexant was well tolerated. There was no evidence of next-day residual effects for suvorexant 10 mg. Suvorexant 50 mg statistically significantly reduced subjective alertness, and suvorexant 100 mg significantly increased reaction time and reduced subjective alertness. There were no statistically significant effects of any suvorexant dose on digit symbol substitution test performance. In Period 5, plasma samples of suvorexant were collected for pharmacokinetic evaluation. The median T(max) was 3 hours and apparent terminal t(½) was 9-13 hours.
Conclusions: In healthy young men without sleep disorders, suvorexant promoted sleep with some evidence of residual effects at the highest doses.
Figures
Similar articles
-
Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant.Neurology. 2012 Dec 4;79(23):2265-74. doi: 10.1212/WNL.0b013e31827688ee. Epub 2012 Nov 28. Neurology. 2012. PMID: 23197752 Clinical Trial.
-
Pharmacodynamic effects of suvorexant and zolpidem on EEG during sleep in healthy subjects.Eur Neuropsychopharmacol. 2016 Oct;26(10):1649-56. doi: 10.1016/j.euroneuro.2016.07.002. Epub 2016 Aug 20. Eur Neuropsychopharmacol. 2016. PMID: 27554636
-
Electroencephalographic power spectral density profile of the orexin receptor antagonist suvorexant in patients with primary insomnia and healthy subjects.Sleep. 2014 Oct 1;37(10):1609-19. doi: 10.5665/sleep.4068. Sleep. 2014. PMID: 25197807 Free PMC article. Clinical Trial.
-
Suvorexant: a dual orexin receptor antagonist for the treatment of sleep onset and sleep maintenance insomnia.Ann Pharmacother. 2015 Apr;49(4):477-83. doi: 10.1177/1060028015570467. Epub 2015 Feb 9. Ann Pharmacother. 2015. PMID: 25667197 Review.
-
Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia.Drugs Today (Barc). 2016 Jan;52(1):29-40. doi: 10.1358/dot.2016.52.1.2439940. Drugs Today (Barc). 2016. PMID: 26937493 Review.
Cited by
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2018 Jan 15;48(1):64-153. Psychopharmacol Bull. 2018. PMID: 29382960 Free PMC article. Review. No abstract available.
-
Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA?CNS Drugs. 2014 Aug;28(8):713-30. doi: 10.1007/s40263-014-0179-x. CNS Drugs. 2014. PMID: 24942635 Review.
-
Acute cognitive effects of the hypocretin receptor antagonist almorexant relative to zolpidem and placebo: a randomized clinical trial.Sleep. 2020 Oct 13;43(10):zsaa080. doi: 10.1093/sleep/zsaa080. Sleep. 2020. PMID: 32303763 Free PMC article. Clinical Trial.
-
Orexins, Sleep, and Blood Pressure.Curr Hypertens Rep. 2018 Jul 10;20(9):79. doi: 10.1007/s11906-018-0879-6. Curr Hypertens Rep. 2018. PMID: 29992504 Free PMC article. Review.
-
Non-24-hour sleep-wake disorder successfully treated with the combination of ramelteon and suvorexant in a case of autism spectrum disorder.Neuropsychopharmacol Rep. 2020 Dec;40(4):383-387. doi: 10.1002/npr2.12142. Epub 2020 Sep 29. Neuropsychopharmacol Rep. 2020. PMID: 32990413 Free PMC article.
References
-
- Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–65. - PubMed
-
- Baumann CR, Bassetti CL. Hypocretins (orexins) and sleep-wake disorders. Lancet Neurol. 2005;4:673–82. - PubMed
-
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical