Mechanisms of pain in osteoarthritis
- PMID: 23372523
- PMCID: PMC3295945
- DOI: 10.1007/s11420-011-9263-7
Mechanisms of pain in osteoarthritis
Keywords: Anesthesiology; Imaging / Radiology; Medicine & Public Health; Orthopedics; Rheumatology; Sports Medicine; Surgical Orthopedics; central sensitization; inflammatory mediators; osteoarthritis; pain; peripheral sensitization.
Figures
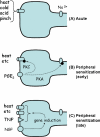
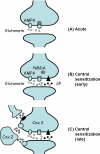
Similar articles
-
Osteoarthritis: The Rheumatologist's Perspective.HSS J. 2012 Feb;8(1):35-6. doi: 10.1007/s11420-011-9253-9. Epub 2011 Dec 23. HSS J. 2012. PMID: 23372525 Free PMC article. No abstract available.
-
The Challenge of Pain for Patients with OA.HSS J. 2012 Feb;8(1):42-4. doi: 10.1007/s11420-011-9254-8. Epub 2012 Jan 27. HSS J. 2012. PMID: 23372528 Free PMC article. No abstract available.
-
Osteoarthritis as a whole joint disease.HSS J. 2012 Feb;8(1):4-6. doi: 10.1007/s11420-011-9248-6. Epub 2012 Feb 23. HSS J. 2012. PMID: 23372516 Free PMC article. No abstract available.
-
Clinical descriptors for the recognition of central sensitization pain in patients with knee osteoarthritis.Disabil Rehabil. 2018 Nov;40(23):2836-2845. doi: 10.1080/09638288.2017.1358770. Epub 2017 Aug 2. Disabil Rehabil. 2018. PMID: 28768437 Review.
-
A Review of Non-Surgical Pain Management in Osteoarthritis.Cureus. 2020 Oct 6;12(10):e10829. doi: 10.7759/cureus.10829. Cureus. 2020. PMID: 33173635 Free PMC article. Review.
Cited by
-
Mechanisms of analgesic effect of mesenchymal stem cells in osteoarthritis pain.World J Stem Cells. 2023 Apr 26;15(4):196-208. doi: 10.4252/wjsc.v15.i4.196. World J Stem Cells. 2023. PMID: 37181003 Free PMC article. Review.
-
Reductions in co-contraction following neuromuscular re-education in people with knee osteoarthritis.BMC Musculoskelet Disord. 2016 Aug 27;17(1):372. doi: 10.1186/s12891-016-1209-2. BMC Musculoskelet Disord. 2016. PMID: 27568007 Free PMC article. Clinical Trial.
-
Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations.Cell. 2021 Sep 2;184(18):4784-4818.e17. doi: 10.1016/j.cell.2021.07.038. Epub 2021 Aug 26. Cell. 2021. PMID: 34450027 Free PMC article.
-
Delineation between different components of chronic pain using dimension reduction - an ASL fMRI study in hand osteoarthritis.Eur J Pain. 2018 Aug;22(7):1245-1254. doi: 10.1002/ejp.1212. Epub 2018 Apr 16. Eur J Pain. 2018. PMID: 29520913 Free PMC article.
-
The role of the central nervous system in osteoarthritis pain and implications for rehabilitation.Curr Rheumatol Rep. 2012 Dec;14(6):576-82. doi: 10.1007/s11926-012-0285-z. Curr Rheumatol Rep. 2012. PMID: 22879060 Review.
References
-
- Raja S, Meyer R, Kingkamp M, JN C. Peripheral neural mechanisms of nociception. In: Wall P, Melzac R, editors. Textbook of pain. Edinburgh: Churchill Livingstone; 1999. p. 11–58.
LinkOut - more resources
Full Text Sources

