Premature and accelerated aging: HIV or HAART?
- PMID: 23372574
- PMCID: PMC3556597
- DOI: 10.3389/fgene.2012.00328
Premature and accelerated aging: HIV or HAART?
Abstract
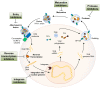
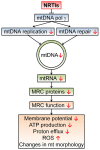
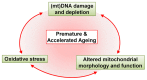
Highly active antiretroviral therapy (HAART) has significantly increased life expectancy of the human immunodeficiency virus (HIV)-positive population. Nevertheless, the average lifespan of HIV-patients remains shorter compared to uninfected individuals. Immunosenescence, a current explanation for this difference invokes heavily on viral stimulus despite HAART efficiency in viral suppression. We propose here that the premature and accelerated aging of HIV-patients can also be caused by adverse effects of antiretroviral drugs, specifically those that affect the mitochondria. The nucleoside reverse transcriptase inhibitor (NRTI) antiretroviral drug class for instance, is known to cause depletion of mitochondrial DNA via inhibition of the mitochondrial specific DNA polymerase-γ. Besides NRTIs, other antiretroviral drug classes such as protease inhibitors also cause severe mitochondrial damage by increasing oxidative stress and diminishing mitochondrial function. We also discuss important areas for future research and argue in favor of the use of Caenorhabditis elegans as a novel model system for studying these effects.
Keywords: C. elegans; HAART; HIV; NRTI; antiretroviral; immunosenescence; mitochondria; premature and accelerated aging.
Figures
Similar articles
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
-
Aging and HIV/AIDS: pathogenetic role of therapeutic side effects.Lab Invest. 2014 Feb;94(2):120-8. doi: 10.1038/labinvest.2013.142. Epub 2013 Dec 16. Lab Invest. 2014. PMID: 24336070 Free PMC article. Review.
-
Nucleoside inhibitors of human immunodeficiency virus type 1 reverse transcriptase.Curr Top Med Chem. 2004;4(9):895-919. doi: 10.2174/1568026043388484. Curr Top Med Chem. 2004. PMID: 15134548 Review.
-
Impact of nucleoside reverse transcriptase inhibitors on mitochondria in human immunodeficiency virus type 1-infected children receiving highly active antiretroviral therapy.Antimicrob Agents Chemother. 2007 Dec;51(12):4236-42. doi: 10.1128/AAC.00893-07. Epub 2007 Sep 24. Antimicrob Agents Chemother. 2007. PMID: 17893156 Free PMC article.
-
Treatment with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children is associated with a sustained effect on growth.Pediatrics. 2002 Feb;109(2):E25. doi: 10.1542/peds.109.2.e25. Pediatrics. 2002. PMID: 11826235
Cited by
-
The struggle of a good friend getting old: cellular senescence in viral responses and therapy.EMBO Rep. 2021 Apr 7;22(4):e52243. doi: 10.15252/embr.202052243. Epub 2021 Mar 18. EMBO Rep. 2021. PMID: 33734564 Free PMC article. Review.
-
HIV Associated Neurodegenerative Disorders: A New Perspective on the Role of Lipid Rafts in Gp120-Mediated Neurotoxicity.Curr HIV Res. 2018;16(4):258-269. doi: 10.2174/1570162X16666181003144740. Curr HIV Res. 2018. PMID: 30280668 Free PMC article. Review.
-
Kinetics of TTV Loads in Peripheral Blood Mononuclear Cells of Early Treated Acute HIV Infections.Viruses. 2023 Sep 15;15(9):1931. doi: 10.3390/v15091931. Viruses. 2023. PMID: 37766337 Free PMC article.
-
Sex-specific neurogenic deficits and neurocognitive disorders in middle-aged HIV-1 Tg26 transgenic mice.Brain Behav Immun. 2019 Aug;80:488-499. doi: 10.1016/j.bbi.2019.04.029. Epub 2019 Apr 16. Brain Behav Immun. 2019. PMID: 30999016 Free PMC article.
-
Disease drivers of aging.Ann N Y Acad Sci. 2016 Dec;1386(1):45-68. doi: 10.1111/nyas.13299. Ann N Y Acad Sci. 2016. PMID: 27943360 Free PMC article. Review.
References
-
- Addo M. G., Cossard R., Pichard D., Obiri-Danso K., Rötig A., Delahodde A. (2010). Caenorhabditis elegans, a pluricellular model organism to screen new genes involved in mitochondrial genome maintenance. Biochim. Biophys. Acta 1802 765–773 - PubMed
-
- Anthony I. C., Ramage S. N., Carnie F. W., Simmonds P., Bell J. E. (2006). Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 111 529–538 - PubMed
-
- Apostolova N., Blas-Garcí A., Esplugues J. V. (2011a). Mitochondrial interference by anti-HIV drugs: mechanisms beyond Pol-γ inhibition. Trends Pharmacol. Sci. 32 715–725 - PubMed
-
- Apostolova N., Gomez-sucerquia L. J., Gortat A., Blas-garcia A., Esplugues J. V. (2011b). Autophagy as a rescue mechanism in efavirenz-induced mitochondrial dysfunction. a lesson from hepatic cells. Autophagy 7 1–3 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

