PPAR-γ activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic β-cells
- PMID: 23372643
- PMCID: PMC3553172
- DOI: 10.1371/journal.pone.0050128
PPAR-γ activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic β-cells
Abstract
Background: It has been reported that peroxisome proliferator-activated receptor (PPAR)-γ and their synthetic ligands have direct effects on pancreatic β-cells. We investigated whether PPAR-γ activation stimulates insulin secretion through the up-regulation of GPR40 in pancreatic β-cells.
Methods: Rat insulinoma INS-1 cells and primary rat islets were treated with rosiglitazone (RGZ) and/or adenoviral PPAR-γ overexpression. OLETF rats were treated with RGZ.
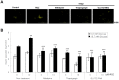
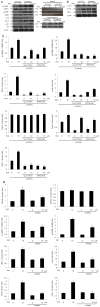
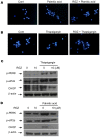
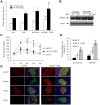
Results: PPAR-γ activation with RGZ and/or adenoviral PPAR-γ overexpression increased free fatty acid (FFA) receptor GPR40 expression, and increased insulin secretion and intracellular calcium mobilization, and was blocked by the PLC inhibitors, GPR40 RNA interference, and GLUT2 RNA interference. As a downstream signaling pathway of intracellular calcium mobilization, the phosphorylated levels of CaMKII and CREB, and the downstream IRS-2 and phospho-Akt were significantly increased. Despite of insulin receptor RNA interference, the levels of IRS-2 and phospho-Akt was still maintained with PPAR-γ activation. In addition, the β-cell specific gene expression, including Pdx-1 and FoxA2, increased in a GPR40- and GLUT2-dependent manner. The levels of GPR40, phosphorylated CaMKII and CREB, and β-cell specific genes induced by RGZ were blocked by GW9662, a PPAR-γ antagonist. Finally, PPAR-γ activation up-regulated β-cell gene expressions through FoxO1 nuclear exclusion, independent of the insulin signaling pathway. Based on immunohistochemical staining, the GLUT2, IRS-2, Pdx-1, and GPR40 were more strongly expressed in islets from RGZ-treated OLETF rats compared to control islets.
Conclusion: These observations suggest that PPAR-γ activation with RGZ and/or adenoviral overexpression increased intracellular calcium mobilization, insulin secretion, and β-cell gene expression through GPR40 and GLUT2 gene up-regulation.
Conflict of interest statement
Figures



References
-
- Ferre P (2004) The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes 53 Suppl 1: S43–S50. - PubMed
-
- Santini E, Fallahi P, Ferrari SM, Masoni A, Antonelli A, et al. (2004) Effect of PPAR-gamma activation and inhibition on glucose-stimulated insulin release in INS-1e cells. Diabetes 53 Suppl 3: S79–S83. - PubMed
-
- Kim HI, Cha JY, Kim SY, Kim JW, Roh KJ, et al. (2002) Peroxisomal proliferator-activated receptor-gamma upregulates glucokinase gene expression in beta-cells. Diabetes 51: 676–685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

