Proline: the distribution, frequency, positioning, and common functional roles of proline and polyproline sequences in the human proteome
- PMID: 23372670
- PMCID: PMC3556072
- DOI: 10.1371/journal.pone.0053785
Proline: the distribution, frequency, positioning, and common functional roles of proline and polyproline sequences in the human proteome
Abstract
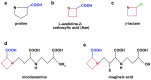
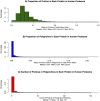
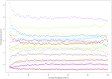
Proline is an anomalous amino acid. Its nitrogen atom is covalently locked within a ring, thus it is the only proteinogenic amino acid with a constrained phi angle. Sequences of three consecutive prolines can fold into polyproline helices, structures that join alpha helices and beta pleats as architectural motifs in protein configuration. Triproline helices are participants in protein-protein signaling interactions. Longer spans of repeat prolines also occur, containing as many as 27 consecutive proline residues. Little is known about the frequency, positioning, and functional significance of these proline sequences. Therefore we have undertaken a systematic bioinformatics study of proline residues in proteins. We analyzed the distribution and frequency of 687,434 proline residues among 18,666 human proteins, identifying single residues, dimers, trimers, and longer repeats. Proline accounts for 6.3% of the 10,882,808 protein amino acids. Of all proline residues, 4.4% are in trimers or longer spans. We detected patterns that influence function based on proline location, spacing, and concentration. We propose a classification based on proline-rich, polyproline-rich, and proline-poor status. Whereas singlet proline residues are often found in proteins that display recurring architectural patterns, trimers or longer proline sequences tend be associated with the absence of repetitive structural motifs. Spans of 6 or more are associated with DNA/RNA processing, actin, and developmental processes. We also suggest a role for proline in Kruppel-type zinc finger protein control of DNA expression, and in the nucleation and translocation of actin by the formin complex.
Conflict of interest statement
Figures

Similar articles
-
A propensity scale for type II polyproline helices (PPII): aromatic amino acids in proline-rich sequences strongly disfavor PPII due to proline-aromatic interactions.Biochemistry. 2012 Jun 26;51(25):5041-51. doi: 10.1021/bi3002924. Epub 2012 Jun 14. Biochemistry. 2012. PMID: 22667692
-
High-resolution structural analysis of mammalian profilin 2a complex formation with two physiological ligands: the formin homology 1 domain of mDia1 and the proline-rich domain of VASP.J Mol Biol. 2008 Jan 4;375(1):270-90. doi: 10.1016/j.jmb.2007.10.050. Epub 2007 Oct 24. J Mol Biol. 2008. PMID: 18001770
-
Polyproline II helix conformation in a proline-rich environment: a theoretical study.Biophys J. 2004 Feb;86(2):731-42. doi: 10.1016/S0006-3495(04)74151-X. Biophys J. 2004. PMID: 14747311 Free PMC article.
-
3-Substituted prolines: from synthesis to structural applications, from peptides to foldamers.Molecules. 2013 Feb 19;18(2):2307-27. doi: 10.3390/molecules18022307. Molecules. 2013. PMID: 23429346 Free PMC article. Review.
-
Structural basis for the role in protein folding of conserved proline-rich regions in cytochromes P450.Toxicol Appl Pharmacol. 2004 Sep 15;199(3):305-15. doi: 10.1016/j.taap.2003.11.030. Toxicol Appl Pharmacol. 2004. PMID: 15364546 Review.
Cited by
-
Ranacyclin-NF, a Novel Bowman-Birk Type Protease Inhibitor from the Skin Secretion of the East Asian Frog, Pelophylax nigromaculatus.Biology (Basel). 2020 Jul 2;9(7):149. doi: 10.3390/biology9070149. Biology (Basel). 2020. PMID: 32630758 Free PMC article.
-
Proline codon pair selection determines ribosome pausing strength and translation efficiency in bacteria.Commun Biol. 2021 May 17;4(1):589. doi: 10.1038/s42003-021-02115-z. Commun Biol. 2021. PMID: 34002016 Free PMC article.
-
Identification of Intrinsically Disordered Proteins and Regions in a Non-Model Insect Species Ostrinia nubilalis (Hbn.).Biomolecules. 2022 Apr 18;12(4):592. doi: 10.3390/biom12040592. Biomolecules. 2022. PMID: 35454181 Free PMC article.
-
Toward predicting silver ion binding in proteins.Chem Commun (Camb). 2025 Apr 1;61(28):5309-5312. doi: 10.1039/d4cc06612j. Chem Commun (Camb). 2025. PMID: 40079190 Free PMC article.
-
Site-Specific Incorporation of Fluorinated Prolines into Proteins and Their Impact on Neighbouring Residues.Chemistry. 2025 Jan 27;31(6):e202403718. doi: 10.1002/chem.202403718. Epub 2024 Dec 30. Chemistry. 2025. PMID: 39661394
References
-
- Rubenstein E (2000) Biologic effects of and clinical disorders caused by nonprotein amino acids. Medicine 79: 80–89. - PubMed
-
- Bell EA (2003) Nonprotein amino acids of plants: significance in medicine, nutrition, and agriculture. Journal of agricultural and food chemistry 51: 2854–2865. - PubMed
-
- MacArthur MW, Thornton JM (1991) Influence of proline residues on protein conformation. Journal of molecular biology 218: 397–412. - PubMed
-
- Baldwin RL (2008) The search for folding intermediates and the mechanism of protein folding. Annu Rev Biophys 37: 1–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

