Inhibition of P-glycoprotein by HIV protease inhibitors increases intracellular accumulation of berberine in murine and human macrophages
- PMID: 23372711
- PMCID: PMC3553168
- DOI: 10.1371/journal.pone.0054349
Inhibition of P-glycoprotein by HIV protease inhibitors increases intracellular accumulation of berberine in murine and human macrophages
Abstract
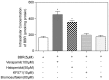
Background: HIV protease inhibitor (PI)-induced inflammatory response in macrophages is a major risk factor for cardiovascular diseases. We have previously reported that berberine (BBR), a traditional herbal medicine, prevents HIV PI-induced inflammatory response through inhibiting endoplasmic reticulum (ER) stress in macrophages. We also found that HIV PIs significantly increased the intracellular concentrations of BBR in macrophages. However, the underlying mechanisms of HIV PI-induced BBR accumulation are unknown. This study examined the role of P-glycoprotein (P-gp) in HIV PI-mediated accumulation of BBR in macrophages.
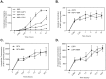
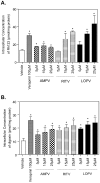
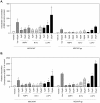
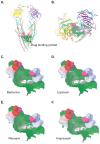
Methodology and principal findings: Cultured mouse RAW264.7 macrophages, human THP-1-derived macrophages, Wild type MDCK (MDCK/WT) and human P-gp transfected (MDCK/P-gp) cells were used in this study. The intracellular concentration of BBR was determined by HPLC. The activity of P-gp was assessed by measuring digoxin and rhodamine 123 (Rh123) efflux. The interaction between P-gp and BBR or HIV PIs was predicated by Glide docking using Schrodinger program. The results indicate that P-gp contributed to the efflux of BBR in macrophages. HIV PIs significantly increased BBR concentrations in macrophages; however, BBR did not alter cellular HIV PI concentrations. Although HIV PIs did not affect P-gp expression, P-gp transport activities were significantly inhibited in HIV PI-treated macrophages. Furthermore, the molecular docking study suggests that both HIV PIs and BBR fit the binding pocket of P-gp, and HIV PIs may compete with BBR to bind P-gp.
Conclusion and significance: HIV PIs increase the concentration of BBR by modulating the transport activity of P-gp in macrophages. Understanding the cellular mechanisms of potential drug-drug interactions is critical prior to applying successful combinational therapy in the clinic.
Conflict of interest statement
Figures

Similar articles
-
HIV-protease inhibitors contribute to P-glycoprotein efflux function defect in peripheral blood lymphocytes from HIV-positive patients receiving HAART.J Acquir Immune Defic Syndr. 2001 Aug 1;27(4):321-30. doi: 10.1097/00126334-200108010-00001. J Acquir Immune Defic Syndr. 2001. PMID: 11468419
-
Berberine inhibits HIV protease inhibitor-induced inflammatory response by modulating ER stress signaling pathways in murine macrophages.PLoS One. 2010 Feb 9;5(2):e9069. doi: 10.1371/journal.pone.0009069. PLoS One. 2010. PMID: 20161729 Free PMC article.
-
Pharmacokinetic interactions between 20(S)-ginsenoside Rh2 and the HIV protease inhibitor ritonavir in vitro and in vivo.Acta Pharmacol Sin. 2013 Oct;34(10):1349-58. doi: 10.1038/aps.2013.69. Epub 2013 Jul 29. Acta Pharmacol Sin. 2013. PMID: 23892274 Free PMC article.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
-
Combination of protease inhibitors for the treatment of HIV-1-infected patients: a review of pharmacokinetics and clinical experience.Antivir Ther. 2001 Dec;6(4):201-29. Antivir Ther. 2001. PMID: 11878403 Review.
Cited by
-
Differential Effect of Simulated Microgravity on the Cellular Uptake of Small Molecules.Pharmaceutics. 2024 Sep 14;16(9):1211. doi: 10.3390/pharmaceutics16091211. Pharmaceutics. 2024. PMID: 39339247 Free PMC article.
-
CUSP9* treatment protocol for recurrent glioblastoma: aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide.Oncotarget. 2014 Sep 30;5(18):8052-82. doi: 10.18632/oncotarget.2408. Oncotarget. 2014. PMID: 25211298 Free PMC article.
-
Berberine inhibits free fatty acid and LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes.PLoS One. 2020 May 1;15(5):e0232630. doi: 10.1371/journal.pone.0232630. eCollection 2020. PLoS One. 2020. PMID: 32357187 Free PMC article.
-
Biological Distribution after Oral Administration of Radioiodine-Labeled Acetaminophen to Estimate Gastrointestinal Absorption Function via OATPs, OATs, and/or MRPs.Pharmaceutics. 2023 Feb 2;15(2):497. doi: 10.3390/pharmaceutics15020497. Pharmaceutics. 2023. PMID: 36839818 Free PMC article.
-
Interaction between post-tumor inflammation and vascular smooth muscle cell dysfunction in sepsis-induced cardiomyopathy.Front Immunol. 2025 Apr 10;16:1560717. doi: 10.3389/fimmu.2025.1560717. eCollection 2025. Front Immunol. 2025. PMID: 40276499 Free PMC article.
References
-
- Zhou H, Pandak WM Jr, Lyall V, Natarajan R, Hylemon PB (2005) HIV protease inhibitors activate the unfolded protein response in macrophages: implication for atherosclerosis and cardiovascular disease. Mol Pharmacol 68: 690–700. - PubMed
-
- Zhou H, Jarujaron S, Gurley EC, Chen L, Ding H, et al. (2007) HIV protease inhibitors increase TNF-alpha and IL-6 expression in macrophages: involvement of the RNA-binding protein HuR. Atherosclerosis 195: e134–143. - PubMed
-
- Parker RA, Flint OP, Mulvey R, Elosua C, Wang F, et al. (2005) Endoplasmic reticulum stress links dyslipidemia to inhibition of proteasome activity and glucose transport by HIV protease inhibitors Author. Mol Pharmacol 67: 1909–1919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

