From principle to practice: bridging the gap in patient profiling
- PMID: 23372761
- PMCID: PMC3556038
- DOI: 10.1371/journal.pone.0054728
From principle to practice: bridging the gap in patient profiling
Abstract
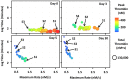
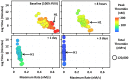
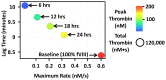
The standard clinical coagulation assays, activated partial thromboplastin time (aPTT) and prothrombin time (PT) cannot predict thrombotic or bleeding risk. Since thrombin generation is central to haemorrhage control and when unregulated, is the likely cause of thrombosis, thrombin generation assays (TGA) have gained acceptance as "global assays" of haemostasis. These assays generate an enormous amount of data including four key thrombin parameters (lag time, maximum rate, peak and total thrombin) that may change to varying degrees over time in longitudinal studies. Currently, each thrombin parameter is averaged and presented individually in a table, bar graph or box plot; no method exists to visualize comprehensive thrombin generation data over time. To address this need, we have created a method that visualizes all four thrombin parameters simultaneously and can be animated to evaluate how thrombin generation changes over time. This method uses all thrombin parameters to intrinsically rank individuals based on their haemostatic status. The thrombin generation parameters can be derived empirically using TGA or simulated using computational models (CM). To establish the utility and diverse applicability of our method we demonstrate how warfarin therapy (CM), factor VIII prophylaxis for haemophilia A (CM), and pregnancy (TGA) affects thrombin generation over time. The method is especially suited to evaluate an individual's thrombotic and bleeding risk during "normal" processes (e.g pregnancy or aging) or during therapeutic challenges to the haemostatic system. Ultimately, our method is designed to visualize individualized patient profiles which are becoming evermore important as personalized medicine strategies become routine clinical practice.
Conflict of interest statement
Figures


Similar articles
-
Warfarin monitoring with viscoelastic haemostatic assays, thrombin generation, coagulation factors and correlations to Owren and Quick prothrombin time.Scand J Clin Lab Invest. 2018 Sep;78(5):358-364. doi: 10.1080/00365513.2018.1474492. Epub 2018 May 23. Scand J Clin Lab Invest. 2018. PMID: 29792060
-
Anticoagulants and the propagation phase of thrombin generation.PLoS One. 2011;6(11):e27852. doi: 10.1371/journal.pone.0027852. Epub 2011 Nov 18. PLoS One. 2011. PMID: 22125631 Free PMC article.
-
Contributions of procoagulants and anticoagulants to the international normalized ratio and thrombin generation assay in patients treated with warfarin: potential role of protein Z as a powerful determinant of coagulation assays.Thromb Res. 2013 Jul;132(1):e70-5. doi: 10.1016/j.thromres.2013.05.015. Epub 2013 Jun 13. Thromb Res. 2013. PMID: 23769659
-
Standardization and clinical utility of thrombin-generation assays.Semin Thromb Hemost. 2008 Oct;34(7):670-82. doi: 10.1055/s-0028-1104546. Epub 2008 Dec 15. Semin Thromb Hemost. 2008. PMID: 19085768 Review.
-
Thrombin generation testing for monitoring hemophilia treatment: a clinical perspective.Semin Thromb Hemost. 2010 Oct;36(7):780-90. doi: 10.1055/s-0030-1265295. Epub 2010 Oct 26. Semin Thromb Hemost. 2010. PMID: 20978999 Review.
Cited by
-
A Prospective Observational Study on Multiplate®-, ROTEM®- and Thrombin Generation Examinations Before and Early After Implantation of a Left Ventricular Assist Device (LVAD).Front Med (Lausanne). 2022 Feb 25;9:760816. doi: 10.3389/fmed.2022.760816. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35280873 Free PMC article.
-
Synthetic data in medicine: Legal and ethical considerations for patient profiling.Comput Struct Biotechnol J. 2025 May 29;28:190-198. doi: 10.1016/j.csbj.2025.05.026. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40520252 Free PMC article.
-
Modeling thrombin generation: plasma composition based approach.J Thromb Thrombolysis. 2014 Jan;37(1):32-44. doi: 10.1007/s11239-013-1006-9. J Thromb Thrombolysis. 2014. PMID: 24214371 Review.
-
Regulation of Early Steps of GPVI Signal Transduction by Phosphatases: A Systems Biology Approach.PLoS Comput Biol. 2015 Nov 19;11(11):e1004589. doi: 10.1371/journal.pcbi.1004589. eCollection 2015 Nov. PLoS Comput Biol. 2015. PMID: 26584182 Free PMC article.
-
Global assays of hemostasis.Curr Opin Hematol. 2014 Sep;21(5):395-403. doi: 10.1097/MOH.0000000000000074. Curr Opin Hematol. 2014. PMID: 25054908 Free PMC article. Review.
References
-
- Nesheim ME, Taswell JB, Mann KG (1979) The contribution of bovine Factor V and Factor Va to the activity of prothrombinase. J Biol Chem 254: 10952–10962. - PubMed
-
- Butenas S, Branda RF, van't Veer C, Cawthern KM, Mann KG (2001) Platelets and phospholipids in tissue factor-initiated thrombin generation. Thromb Haemost 86: 660–667. - PubMed
-
- Kisiel W, Canfield WM, Ericsson LH, Davie EW (1977) Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry 16: 5824–5831. - PubMed
-
- Shatos MA, Orfeo T, Doherty JM, Penar PL, Collen D, et al. (1995) Alpha-thrombin stimulates urokinase production and DNA synthesis in cultured human cerebral microvascular endothelial cells. Arterioscler Thromb Vasc Biol 15: 903–911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

