Cis and trans regulatory mechanisms control AP2-mediated B cell receptor endocytosis via select tyrosine-based motifs
- PMID: 23372794
- PMCID: PMC3553015
- DOI: 10.1371/journal.pone.0054938
Cis and trans regulatory mechanisms control AP2-mediated B cell receptor endocytosis via select tyrosine-based motifs
Abstract
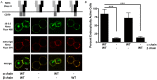
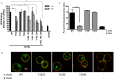
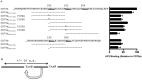
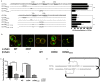
Following antigen recognition, B cell receptor (BCR)-mediated endocytosis is the first step of antigen processing and presentation to CD4+ T cells, a crucial component of the initiation and control of the humoral immune response. Despite this, the molecular mechanism of BCR internalization is poorly understood. Recently, studies of activated B cell-like diffuse large B cell lymphoma (ABC DLBCL) have shown that mutations within the BCR subunit CD79b leads to increased BCR surface expression, suggesting that CD79b may control BCR internalization. Adaptor protein 2 (AP2) is the major mediator of receptor endocytosis via clathrin-coated pits. The BCR contains five putative AP2-binding YxxØ motifs, including four that are present within two immunoreceptor tyrosine-based activation motifs (ITAMs). Using a combination of in vitro and in situ approaches, we establish that the sole mediator of AP2-dependent BCR internalization is the membrane proximal ITAM YxxØ motif in CD79b, which is a major target of mutation in ABC DLBCL. In addition, we establish that BCR internalization can be regulated at a minimum of two different levels: regulation of YxxØ AP2 binding in cis by downstream ITAM-embedded DCSM and QTAT regulatory elements and regulation in trans by the partner cytoplasmic domain of the CD79 heterodimer. Beyond establishing the basic rules governing BCR internalization, these results illustrate an underappreciated role for ITAM residues in controlling clathrin-dependent endocytosis and highlight the complex mechanisms that control the activity of AP2 binding motifs in this receptor system.
Conflict of interest statement
Figures





Similar articles
-
Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma.Nature. 2010 Jan 7;463(7277):88-92. doi: 10.1038/nature08638. Nature. 2010. PMID: 20054396 Free PMC article.
-
Activation of Self-Incompatibility Signaling in Transgenic Arabidopsis thaliana Is Independent of AP2-Based Clathrin-Mediated Endocytosis.G3 (Bethesda). 2018 Jul 2;8(7):2231-2239. doi: 10.1534/g3.118.200231. G3 (Bethesda). 2018. PMID: 29720392 Free PMC article.
-
Clathrin promotes incorporation of cargo into coated pits by activation of the AP2 adaptor micro2 kinase.J Cell Biol. 2003 Oct 27;163(2):231-6. doi: 10.1083/jcb.200304079. J Cell Biol. 2003. PMID: 14581451 Free PMC article.
-
The ins and outs of getting in: structures and signals that enhance BCR or Fc receptor-mediated antigen presentation.Immunopharmacology. 2000 Sep;49(3):227-40. doi: 10.1016/s0162-3109(00)00255-1. Immunopharmacology. 2000. PMID: 10996020 Review.
-
Cargo recognition during clathrin-mediated endocytosis: a team effort.Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review.
Cited by
-
Profile of Polatuzumab Vedotin in the Treatment of Patients with Relapsed/Refractory Non-Hodgkin Lymphoma: A Brief Report on the Emerging Clinical Data.Onco Targets Ther. 2020 Jun 8;13:5123-5133. doi: 10.2147/OTT.S219449. eCollection 2020. Onco Targets Ther. 2020. PMID: 32606733 Free PMC article. Review.
-
The Other Function: Class II-Restricted Antigen Presentation by B Cells.Front Immunol. 2017 Mar 23;8:319. doi: 10.3389/fimmu.2017.00319. eCollection 2017. Front Immunol. 2017. PMID: 28386257 Free PMC article. Review.
-
Endophilin A2 regulates B-cell endocytosis and is required for germinal center and humoral responses.EMBO Rep. 2021 Sep 6;22(9):e51328. doi: 10.15252/embr.202051328. Epub 2021 Jul 29. EMBO Rep. 2021. PMID: 34323351 Free PMC article.
-
Characterization of interactions within the Igα/Igβ transmembrane domains of the human B-cell receptor provides insights into receptor assembly.J Biol Chem. 2022 May;298(5):101843. doi: 10.1016/j.jbc.2022.101843. Epub 2022 Mar 18. J Biol Chem. 2022. PMID: 35307351 Free PMC article.
-
The ap-2 clathrin adaptor mediates endocytosis of an inhibitory killer cell Ig-like receptor in human NK cells.J Immunol. 2014 Nov 1;193(9):4675-83. doi: 10.4049/jimmunol.1303406. Epub 2014 Sep 19. J Immunol. 2014. PMID: 25238755 Free PMC article.
References
-
- Lanzavecchia A (1985) Antigen-specific interaction between T and B cells. Nature 314: 537–539. - PubMed
-
- Chesnut RW, Colon SM, Grey HM (1982) Requirements for the processing of antigens by antigen-presenting B cells. I. Functional comparison of B cell tumors and macrophages. J Immunol 129: 2382–2388. - PubMed
-
- Grey HM, Colon SM, Chesnut RW (1982) Requirements for the processing of antigen by antigen-presenting B cells. II. Biochemical comparison of the fate of antigen in B cell tumors and macrophages. J Immunol 129: 2389–2395. - PubMed
-
- Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M (1990) Molecular components of the B-cell antigen receptor complex of the IgM class. Nature 343: 760–762. - PubMed
-
- Yamanashi Y, Kakiuchi T, Mizuguchi J, Yamamoto T, Toyoshima K (1991) Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science 251: 192–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

