Effectiveness of efavirenz-based regimens in young HIV-infected children treated for tuberculosis: a treatment option for resource-limited settings
- PMID: 23372824
- PMCID: PMC3555823
- DOI: 10.1371/journal.pone.0055111
Effectiveness of efavirenz-based regimens in young HIV-infected children treated for tuberculosis: a treatment option for resource-limited settings
Abstract
Background: Antiretroviral treatment (ART) options for young children co-infected with HIV and tuberculosis are limited in resource-poor settings due to limited data on the use of efavirenz (EFV). Using available pharmacokinetic data, an EFV dosing schedule was developed for young co-infected children and implemented as the standard of care at Macha Hospital in Southern Province, Zambia. Treatment outcomes in children younger than 3 years of age or weighing less than 10 kg receiving either EFV-based ART plus anti-tuberculous treatment or nevirapine-based (NVP) ART were compared.
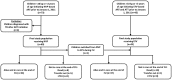
Methods: Treatment outcomes were measured in a cohort of HIV-infected children seeking care at Macha Hospital in rural Zambia from 2007 to 2010. Information on the diagnosis and treatment of tuberculosis was abstracted from medical records.
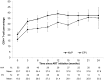
Results: Forty-five children treated for tuberculosis initiated an EFV-based regimen and 69 children initiated a NVP-based regimen, 7 of whom also were treated for tuberculosis. Children receiving both regimens were comparable in age, but children receiving EFV started ART with a lower CD4(+) T-cell percentage and weight-for-age z-score. Children receiving EFV experienced increases in both CD4(+) T-cell percentage and weight-for-age z-score during follow-up, such that levels were comparable to children receiving NVP after two years of ART. Cumulative survival after 12 months of ART did not differ between groups (NVP:87%;EFV:80%;p = 0.25). Eleven children experienced virologic failure during follow-up.The adjusted hazard ratio of virologic failure comparing EFV to NVP was 0.25 (95% CI:0.05,1.24) and 0.13 (95% CI:0.03,0.62) using thresholds of 5000 and 400 copies/mL, respectively.Five children receiving EFV were reported to have had convulsions after ART initiation compared to only one child receiving NVP (p = 0.04).
Conclusions: Despite poorer health at ART initiation, children treated for tuberculosis and receiving EFV-based regimens showed significant improvements comparable to children receiving NVP-based regimens. EFV-based regimens should be considered for young HIV-infected children co-infected with tuberculosis in resource-limited settings.
Conflict of interest statement
Figures



References
-
- UNAIDS (2012) Together We Will End AIDS. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS. - PubMed
-
- Walls T, Shingadia D (2004) Global epidemiology of paediatric tuberculosis. J Infect 48: 13–22. - PubMed
-
- Jeena PM, Coovadia HM, Thula SA, Blythe D, Buckels NJ, et al. (1998) Persistent and chronic lung disease in HIV-1 infected and uninfected African children. AIDS 12: 1185–1193. - PubMed
-
- Chintu C, Mudenda V, Lucas S, Nunn A, Lishimpi K, et al. (2002) Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet 360: 985–990. - PubMed
-
- Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, et al. (1995) Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med 151: 129–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

