Peroxisome proliferator-activated receptor gamma (PPARγ) regulates thrombospondin-1 and Nox4 expression in hypoxia-induced human pulmonary artery smooth muscle cell proliferation
- PMID: 23372933
- PMCID: PMC3555419
- DOI: 10.4103/2045-8932.105037
Peroxisome proliferator-activated receptor gamma (PPARγ) regulates thrombospondin-1 and Nox4 expression in hypoxia-induced human pulmonary artery smooth muscle cell proliferation
Abstract
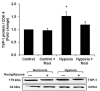
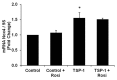
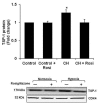
Transforming growth factor-β1 (TGF- β1) and thrombospondin-1 (TSP-1) are hypoxia-responsive mitogens that promote vascular smooth muscle cell (SMC) proliferation, a critical event in the pathogenesis of pulmonary hypertension (PH). We previously demonstrated that hypoxia-induced human pulmonary artery smooth muscle (HPASMC) cell proliferation and expression of the NADPH oxidase subunit, Nox4, were attenuated by the peroxisome proliferator-activated receptor γ (PPARγ) agonist, rosiglitazone. The current study examines the hypothesis that rosiglitazone regulates Nox4 expression and HPASMC proliferation by attenuating TSP-1 signaling. Selected HPASMC were exposed to normoxic or hypoxic (1% O(2)) environments or TSP-1 (0-1 μg/ ml) for 72 hours ± administration of rosiglitazone (10 μM). Cellular proliferation, Nox4, TSP-1, and TGF-β1 expression and reactive oxygen species generation were measured. Mice exposed to hypoxia (10% O(2)) for three weeks were treated with rosiglitazone (10 mg/kg/day) for the final 10 days, and lung TSP-1 expression was examined. Hypoxia increased TSP-1 and TGF-β1 expression and HPASMC proliferation, and neutralizing antibodies to TSP-1 or TGF-β1 attenuated proliferation. Rosiglitazone attenuated hypoxia-induced HPASMC proliferation and increases in mouse lung and HPASMC TSP-1 expression, but failed to reduce increases in TGF-β1 expression or Nox4 expression and activity caused by direct TSP-1 stimulation. Transfecting HPASMC with siRNA to Nox4 attenuated hypoxia- or TSP-1-stimulated HPASMC proliferation. These findings provide novel evidence that TSP-1-mediated Nox4 expression plays a critical role in hypoxia-induced HPASMC proliferation. PPARγ activation with exogenous ligands attenuates TSP-1 expression to reduce Nox4 expression. These results clarify mechanisms of hypoxia-induced SMC proliferation and suggest additional pathways by which PPARγ agonists may regulate critical steps in the pathobiology of PH.
Keywords: Nox4; PPARγ; hypoxia; rosiglitazone; thrombospondin-1.
Conflict of interest statement
Figures


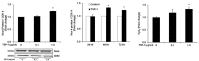


Similar articles
-
PPAR{gamma} regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-{kappa}B.Am J Physiol Lung Cell Mol Physiol. 2010 Oct;299(4):L559-66. doi: 10.1152/ajplung.00090.2010. Epub 2010 Jul 9. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20622120 Free PMC article.
-
NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3.Am J Physiol Lung Cell Mol Physiol. 2009 Mar;296(3):L489-99. doi: 10.1152/ajplung.90488.2008. Epub 2008 Nov 26. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19036873 Free PMC article.
-
Peroxisome proliferator-activated receptor gamma depletion stimulates Nox4 expression and human pulmonary artery smooth muscle cell proliferation.Free Radic Biol Med. 2015 Mar;80:111-20. doi: 10.1016/j.freeradbiomed.2014.12.019. Epub 2014 Dec 31. Free Radic Biol Med. 2015. PMID: 25557278 Free PMC article.
-
Hypoxia downregulates PPARγ via an ERK1/2-NF-κB-Nox4-dependent mechanism in human pulmonary artery smooth muscle cells.Free Radic Biol Med. 2013 Oct;63:151-60. doi: 10.1016/j.freeradbiomed.2013.05.013. Epub 2013 May 15. Free Radic Biol Med. 2013. PMID: 23684777 Free PMC article.
-
The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation.Am J Respir Cell Mol Biol. 2012 Nov;47(5):718-26. doi: 10.1165/rcmb.2011-0418OC. Epub 2012 Aug 16. Am J Respir Cell Mol Biol. 2012. PMID: 22904198 Free PMC article.
Cited by
-
CD47 and thrombospondin-1 regulation of mitochondria, metabolism, and diabetes.Am J Physiol Cell Physiol. 2021 Aug 1;321(2):C201-C213. doi: 10.1152/ajpcell.00175.2021. Epub 2021 Jun 9. Am J Physiol Cell Physiol. 2021. PMID: 34106789 Free PMC article. Review.
-
Effects of chrysin (5,7-dihydroxyflavone) on vascular remodeling in hypoxia-induced pulmonary hypertension in rats.Chin Med. 2015 Feb 18;10:4. doi: 10.1186/s13020-015-0032-2. eCollection 2015. Chin Med. 2015. PMID: 25722740 Free PMC article.
-
Hypoxia mediates mutual repression between microRNA-27a and PPARγ in the pulmonary vasculature.PLoS One. 2013 Nov 14;8(11):e79503. doi: 10.1371/journal.pone.0079503. eCollection 2013. PLoS One. 2013. PMID: 24244514 Free PMC article.
-
NADPH oxidases: redox regulation of cell homeostasis and disease.Physiol Rev. 2025 Jul 1;105(3):1291-1428. doi: 10.1152/physrev.00034.2023. Epub 2025 Jan 15. Physiol Rev. 2025. PMID: 39814410 Free PMC article. Review.
-
Time-dependent PPARγ Modulation of HIF-1α Signaling in Hypoxic Pulmonary Artery Smooth Muscle Cells.Am J Med Sci. 2016 Jul;352(1):71-9. doi: 10.1016/j.amjms.2016.03.019. Epub 2016 Apr 4. Am J Med Sci. 2016. PMID: 27432037 Free PMC article.
References
-
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: Results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–30. - PubMed
-
- Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–65. - PubMed
-
- Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

