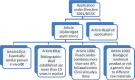
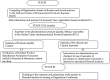
A comprehensive study on regulatory requirements for development and filing of generic drugs globally
- PMID: 23373001
- PMCID: PMC3555014
- DOI: 10.4103/2230-973X.104392
A comprehensive study on regulatory requirements for development and filing of generic drugs globally
Abstract
The regulatory requirements of various countries of the world vary from each other. Therefore, it is challenging for the companies to develop a single drug which can be simultaneously submitted in all the countries for approval. The regulatory strategy for product development is essentially to be established before commencement of developmental work in order to avoid major surprises after submission of the application. The role of the regulatory authorities is to ensure the quality, safety, and efficacy of all medicines in circulation in their country. It not only includes the process of regulating and monitoring the drugs but also the process of manufacturing, distribution, and promotion of it. One of the primary challenges for regulatory authority is to ensure that the pharmaceutical products are developed as per the regulatory requirement of that country. This process involves the assessment of critical parameters during product development.
Keywords: Development; drug; generic; global; regulatory.
Conflict of interest statement
Figures
References
-
- Stephanie Sutton Global Market Boom for Generic Drugs. ON The Electronic Newsletter of Pharmaceutical Technology. 2012. [Last accessed on 2012 Jan 19]. Available from: http://www.pharmtech.com/pharmtech/News/Global-Market-Boom-for-Generic-D... .
-
- Srinivasan R. Indian pharmaceutical industry: Evaluation of current scenario and future trends. [Last accessed on 2012 Jun 10]. Available from: http://www.tejas-iimb.org/interviews/13.php .
-
- Hamrell MR. 2. Vol. 14. California: ON Clinical Research and Regulatory Affairs; 1997. [Last accessed on 2012 Jun 10]. An Update on the Generic Drug Approval Process; pp. 139–54. Available from: http://www.informahealthcare.com/doi/abs/10.3109/10601339709019635?journ... .
-
- Redmond K. The US and European Regulatory Systems: A Comparison: ON JAmbul Care Manage. [Last accessed on 2011 Nov];2004 27:105–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15069987 . - PubMed
-
- [Last accessed on 2012 Apr]. Available from: http://www.fda.gov/AboutFDA/CentersOffices/OrganizationCharts/ucm135674....
LinkOut - more resources
Full Text Sources
Other Literature Sources