Dendritic cell activation, phagocytosis and CD69 expression on cognate T cells are suppressed by n-3 long-chain polyunsaturated fatty acids
- PMID: 23373457
- PMCID: PMC3701185
- DOI: 10.1111/imm.12088
Dendritic cell activation, phagocytosis and CD69 expression on cognate T cells are suppressed by n-3 long-chain polyunsaturated fatty acids
Abstract
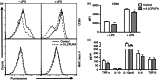
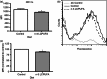
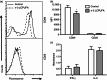
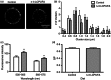
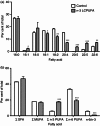
Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are bioactive n-3 long-chain polyunsaturated fatty acids (LCPUFAs) in fish oil that exert immunosuppressive effects. A significant amount of literature shows that n-3 LCPUFAs suppress dendritic cell (DC) function in vitro; however, few studies have determined if the effects are emulated at the animal level. In this study, we first focused on the functional consequences of 5% (weight/weight) fish oil on splenic CD11c(+) DCs. Administration of n-3 LCPUFAs, modelling human pharmacological intake (2% of total kcal from EPA,1·3% from DHA), to C57BL/6 mice for 3 weeks reduced DC surface expression of CD80 by 14% and tumour necrosis factor-α secretion by 29% upon lipopolysaccharide stimulation relative to a control diet. The n-3 LCPUFAs also significantly decreased CD11c(+) surface expression and phagocytosis by 12% compared with the control diet. Antigen presentation studies revealed a 22% decrease in CD69 surface expression on transgenic CD4(+) T lymphocytes activated by DCs from mice fed fish oil. We then determined if the functional changes were mechanistically associated with changes in lipid microdomain clustering or plasma membrane microviscosity with n-3 LCPUFAs, as reported for B and T lymphocytes. Fish oil administration to mice did not influence cholera-toxin induced lipid microdomain clustering or microviscosity, even though EPA and DHA levels were significantly elevated relative to the control diet. Overall, our data show that n-3 LCPUFAs exert immunosuppressive effects on DCs, validating in vitro studies. The results also show that DC microdomain clustering and microviscosity were not changed by the n-3 LCPUFA intervention used in this study.
© 2013 John Wiley & Sons Ltd.
Figures
Similar articles
-
Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y.Am J Clin Nutr. 2001 Mar;73(3):539-48. doi: 10.1093/ajcn/73.3.539. Am J Clin Nutr. 2001. PMID: 11237929 Clinical Trial.
-
Modification of spleen phospholipid fatty acid composition by dietary fish oil and by n-3 fatty acid ethyl esters.J Lipid Res. 1993 Aug;34(8):1423-34. J Lipid Res. 1993. PMID: 8409773
-
Low levels of eicosapentaenoic and docosahexaenoic acids mimic the effects of fish oil upon rat lymphocytes.Life Sci. 1998;62(24):2209-17. doi: 10.1016/s0024-3205(98)00199-4. Life Sci. 1998. PMID: 9627080
-
Myocardial membrane fatty acids and the antiarrhythmic actions of dietary fish oil in animal models.Lipids. 2001;36 Suppl:S111-4. doi: 10.1007/s11745-001-0692-x. Lipids. 2001. PMID: 11837983 Review.
-
N-3 fatty acids from fish oil. Effects on plasma lipoproteins and hypertriglyceridemic patients.Ann N Y Acad Sci. 1993 Jun 14;683:16-34. doi: 10.1111/j.1749-6632.1993.tb35689.x. Ann N Y Acad Sci. 1993. PMID: 8352438 Review.
Cited by
-
Pathways of polyunsaturated fatty acid utilization: implications for brain function in neuropsychiatric health and disease.Brain Res. 2015 Feb 9;1597:220-46. doi: 10.1016/j.brainres.2014.11.059. Epub 2014 Dec 8. Brain Res. 2015. PMID: 25498862 Free PMC article. Review.
-
Influence of Ovophospholipids on Lymphocyte Subsets and Humoral Immune Response in Mice.Molecules. 2025 May 22;30(11):2253. doi: 10.3390/molecules30112253. Molecules. 2025. PMID: 40509141 Free PMC article.
-
Maternal gut microbiome regulates immunity to RSV infection in offspring.J Exp Med. 2021 Nov 1;218(11):e20210235. doi: 10.1084/jem.20210235. Epub 2021 Oct 6. J Exp Med. 2021. PMID: 34613328 Free PMC article.
-
B Cell Activity Is Impaired in Human and Mouse Obesity and Is Responsive to an Essential Fatty Acid upon Murine Influenza Infection.J Immunol. 2017 Jun 15;198(12):4738-4752. doi: 10.4049/jimmunol.1601031. Epub 2017 May 12. J Immunol. 2017. PMID: 28500069 Free PMC article.
-
Effects of parental omega-3 fatty acid intake on offspring microbiome and immunity.PLoS One. 2014 Jan 29;9(1):e87181. doi: 10.1371/journal.pone.0087181. eCollection 2014. PLoS One. 2014. PMID: 24489864 Free PMC article.
References
-
- Han YY, Lai SL, Ko WJ, Chou CH, Lai HS. Effects of fish oil on inflammatory modulation in surgical intensive care unit patients. Nutr Clin Pract. 2012;27:91–8. - PubMed
-
- Luu NT, Madden J, Calder PC, et al. Dietary supplementation with fish oil modifies the ability of human monocytes to induce an inflammatory response. J Nutr. 2007;137:2769–74. - PubMed
-
- Trebble TM, Stroud MA, Wootton SA, et al. High-dose fish oil and antioxidants in Crohn's disease and the response of bone turnover: a randomised controlled trial. Br J Nutr. 2005;94:253–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

