Human metapneumovirus keeps dendritic cells from priming antigen-specific naive T cells
- PMID: 23374037
- PMCID: PMC3701183
- DOI: 10.1111/imm.12083
Human metapneumovirus keeps dendritic cells from priming antigen-specific naive T cells
Abstract
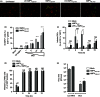
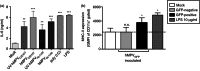
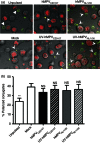
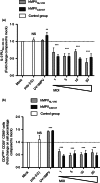
Human metapneumovirus (hMPV) is the second most common cause of acute lower respiratory tract infections in children, causing a significant public health burden worldwide. Given that hMPV can repeatedly infect the host without major antigenic changes, it has been suggested that hMPV may have evolved molecular mechanisms to impair host adaptive immunity and, more specifically, T-cell memory. Recent studies have shown that hMPV can interfere with superantigen-induced T-cell activation by infecting conventional dendritic cells (DCs). Here, we show that hMPV infects mouse DCs in a restricted manner and induces moderate maturation. Nonetheless, hMPV-infected DCs are rendered inefficient at activating naive antigen-specific CD4(+) T cells (OT-II), which not only display reduced proliferation, but also show a marked reduction in surface activation markers and interleukin-2 secretion. Decreased T-cell activation was not mediated by interference with DC-T-cell immunological synapse formation as recently described for the human respiratory syncytial virus (hRSV), but rather by soluble factors secreted by hMPV-infected DCs. These data suggest that although hMPV infection is restricted within DCs, it is sufficient to interfere with their capacity to activate naive T cells. Altogether, by interfering with DC function and productive priming of antigen-inexperienced T cells, hMPV could impair the generation of long-term immunity.
© 2013 John Wiley & Sons Ltd.
Figures

Similar articles
-
Effects of human respiratory syncytial virus, metapneumovirus, parainfluenza virus 3 and influenza virus on CD4+ T cell activation by dendritic cells.PLoS One. 2010 Nov 29;5(11):e15017. doi: 10.1371/journal.pone.0015017. PLoS One. 2010. PMID: 21124776 Free PMC article.
-
Human metapneumovirus SH and G glycoproteins inhibit macropinocytosis-mediated entry into human dendritic cells and reduce CD4+ T cell activation.J Virol. 2014 Jun;88(11):6453-69. doi: 10.1128/JVI.03261-13. Epub 2014 Mar 26. J Virol. 2014. PMID: 24672038 Free PMC article.
-
Low CCR7-mediated migration of human monocyte derived dendritic cells in response to human respiratory syncytial virus and human metapneumovirus.PLoS Pathog. 2011 Jun;7(6):e1002105. doi: 10.1371/journal.ppat.1002105. Epub 2011 Jun 23. PLoS Pathog. 2011. PMID: 21731495 Free PMC article.
-
Aberrant T cell immunity triggered by human Respiratory Syncytial Virus and human Metapneumovirus infection.Virulence. 2017 Aug 18;8(6):685-704. doi: 10.1080/21505594.2016.1265725. Epub 2016 Dec 2. Virulence. 2017. PMID: 27911218 Free PMC article. Review.
-
Human Metapneumovirus: Mechanisms and Molecular Targets Used by the Virus to Avoid the Immune System.Front Immunol. 2018 Oct 24;9:2466. doi: 10.3389/fimmu.2018.02466. eCollection 2018. Front Immunol. 2018. PMID: 30405642 Free PMC article. Review.
Cited by
-
Dendritic cells in human Pneumovirus and Metapneumovirus infections.Viruses. 2013 Jun 20;5(6):1553-70. doi: 10.3390/v5061553. Viruses. 2013. PMID: 23787776 Free PMC article. Review.
-
Clinical significance of human metapneumovirus detection in critically ill adults with lower respiratory tract infections.Ann Intensive Care. 2023 Mar 20;13(1):21. doi: 10.1186/s13613-023-01117-w. Ann Intensive Care. 2023. PMID: 36940047 Free PMC article.
-
BCG-Based Vaccines Elicit Antigen-Specific Adaptive and Trained Immunity against SARS-CoV-2 and Andes orthohantavirus.Vaccines (Basel). 2022 May 4;10(5):721. doi: 10.3390/vaccines10050721. Vaccines (Basel). 2022. PMID: 35632475 Free PMC article.
-
Herpes Simplex Virus Evasion of Early Host Antiviral Responses.Front Cell Infect Microbiol. 2019 Apr 30;9:127. doi: 10.3389/fcimb.2019.00127. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31114761 Free PMC article. Review.
-
Contribution of NKT cells to the immune response and pathogenesis triggered by respiratory viruses.Virulence. 2020 Dec;11(1):580-593. doi: 10.1080/21505594.2020.1770492. Virulence. 2020. PMID: 32463330 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

