The CRTH2 agonist Pyl A prevents lipopolysaccharide-induced fetal death but induces preterm labour
- PMID: 23374103
- PMCID: PMC3701182
- DOI: 10.1111/imm.12085
The CRTH2 agonist Pyl A prevents lipopolysaccharide-induced fetal death but induces preterm labour
Abstract
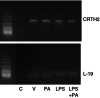
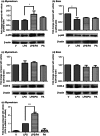
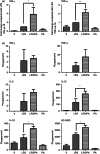
We have previously demonstrated that the anti-inflammatory prostaglandin 15-deoxy-Δ 12,14-prostaglandin J(2) (15dPGJ(2)) delays inflammation-induced preterm labour in the mouse and improves pup survival through the inhibition of nuclear factor-κB (NF-κB) by a mechanism yet to be elucidated. 15dPGJ(2) is an agonist of the second prostaglandin D(2) receptor, chemoattractant receptor homologous to the T helper 2 cell (CRTH2). In human T helper cells CRTH2 agonists induce the production of the anti-inflammatory interleukins IL-10 and IL-4. We hypothesized that CRTH2 is involved in the protective effect of 15dPGJ(2) in inflammation-induced preterm labour in the murine model. We therefore studied the effects of a specific small molecule CRTH2 agonist on preterm labour and pup survival. An intrauterine injection of lipopolysaccharide (LPS) was administered to CD1 mice at embryonic day 16, ± CRTH2 agonist/vehicle controls. Mice were killed at 4.5 hr to assess fetal wellbeing and to harvest myometrium and pup brain for analysis of NF-κB, and T helper type 1/2 interleukins. To examine the effects of the CRTH2 agonist on LPS-induced preterm labour, mice were allowed to labour spontaneously. Direct effects of the CRTH2 agonist on uterine contractility were examined ex vivo on contracting myometrial strips. The CRTH2 agonist increased fetal survival from 20 to 100% in LPS-treated mice, and inhibited circular muscle contractility ex vivo. However, it augmented LPS-induced labour and significantly increased myometrial NF-κB, IL-1β, KC-GRO, interferon-γ and tumour necrosis factor-α. This suggests that the action of 15dPGJ(2) is not via CRTH2 and therefore small molecule CRTH2 agonists are not likely to be beneficial for the prevention of inflammation-induced preterm labour.
© 2013 John Wiley & Sons Ltd.
Figures



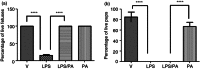

References
-
- Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489–97. - PubMed
-
- Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. - PubMed
-
- Sykes L, MacIntyre D, Teoh T, Bennett P. Targeting immune activation in the prevention of preterm labour. Eur Obstet Gynecol. 2011;6:100–6.
-
- Lindstrom TM, Bennett PR. The role of nuclear factor κB in human labour. Reproduction. 2005;130:569–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

