In vivo activity of nisin A and nisin V against Listeria monocytogenes in mice
- PMID: 23374279
- PMCID: PMC3616995
- DOI: 10.1186/1471-2180-13-23
In vivo activity of nisin A and nisin V against Listeria monocytogenes in mice
Abstract
Background: Lantibiotics are post-translationally modified antimicrobial peptides, of which nisin A is the most extensively studied example. Bioengineering of nisin A has resulted in the generation of derivatives with increased in vitro potency against Gram-positive bacteria. Of these, nisin V (containing a Met21Val change) is noteworthy by virtue of exhibiting enhanced antimicrobial efficacy against a wide range of clinical and food-borne pathogens, including Listeria monocytogenes. However, this increased potency has not been tested in vivo.
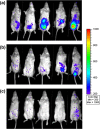
Results: Here we address this issue by assessing the ability of nisin A and nisin V to control a bioluminescent strain of Listeria monocytogenes EGDe in a murine infection model.More specifically, Balb/c mice were infected via the intraperitoneal route at a dose of 1 × 10(5) cfu/animal and subsequently treated intraperitoneally with either nisin V, nisin A or a PBS control. Bioimaging of the mice was carried out on day 3 of the trial. Animals were then sacrificed and levels of infection were quantified in the liver and spleen.
Conclusion: This analysis revealed that nisin V was more effective than Nisin A with respect to controlling infection and therefore merits further investigation with a view to potential chemotherapeutic applications.
Figures


References
-
- Chen H, Hoover DG. Bacteriocins and their food applications. Comprehensive Rev Food Sci Food Safety. 2003;2:82–100. - PubMed
-
- Delves-Broughton J. Nisin and its uses as a food preservative. Food Technol. 1990;44:100–117.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

